Review Article - Interventional Cardiology (2015) Volume 7, Issue 1
Evaluating neoatherosclerosis for risk stratification of very-late DES failure
- Corresponding Author:
- Yasunori Ueda
Cardiovascular Division, Osaka Police Hospital, 10–31 Kitayama-cho,Tennoji-ku, Osaka, 543–0035, Japan
Tel: +81 667 716 051
Fax: +81 667 752 845
E-mail: ueda@oph.gr.jp
Abstract
Drug-eluting stents (DESs) in comparison with bare metal stents (BMSs) have reduced early target lesion revascularization (TLR) through exerting an inhibitory effect n smooth muscle cell hyperplasia but have also increased the risk of stent thr mb sis and TLR after 1 year, in other words, very-late stent failure (VLSF). Neoather scler sis or atherosclerosis progression is thought to be the major mechanism and is regarded as a ‘final common pathway of VLSF.’ Atherosclerosis can be detected by vari us intracoronary imaging modalities; e.g., angioscopy detects it as yell w plaque that has been regarded as vulnerable plaque and is associated with futu e event f acute coronary syndrome. Here, the findings of neoatherosclerosis using int acoronary imaging are reviewed and their relation with the long - term clinical outcome is discussed.
Keywords
angioscopy,drug-eluting stent,very late stent failure,yellow plaque
Neoatherosclerosis at very-late stent failure detected by intracoronary imaging
Drug-eluting stents (DESs) in comparison with bare metal stents (BMSs) have reduced early target lesion revascularization (TLR) by inhibiting smooth muscle cell hyperplasia; however, the incidence of stent thrombosis and TLR after one year, in other words, very-late stent failure (VLSF), was higher in the 1st generation DESs than with BMS [1]. Although the incidence of VLSF was reduced in newer DESs in comparison with 1st generation DESs [2–8], VLSF remains an unsolved problem of DESs and its mechanism has not been clarified.
Atherosclerosis progression or neoatherosclerosis is thought to be one of the mechanisms of VLSF [9] as it has been reported as a probable cause of both very late stent thrombosis [10–13] and restenosis after DES implantation [14–21]. Although the early and late stent failure up to 1 year after implantation involves multifactorial mechanisms including procedure-related ones, stent failure after 1 year is supposed to be caused mainly by atherosclerosis progression or neoatherosclerosis, which is regarded as the ‘final common pathway of VLSF’ [22,23]. Angioscopy, as a tool of macroscopic pathology in living patients, can detect atherosclerosis by yellow plaque. Yellow plaques, especially those of high yellow color grade, are regarded vulnerable plaques and have been associated with future coronary events by many clinical studies [21,24–26]. Although angioscopy used to be performed both in Europe and in the USA, it is now available only in Japan and these devices are manufactured only by Japanese companies.
The culprit lesions of very late stent thrombosis have been shown by case reports to have disrupted yellow plaque by coronary angioscopy [27]. Optical coherence tomography (OCT) studies also revealed that they have advanced neoatherosclerosis of lipidladed neointima and plaque rupture in the majority of cases both after BMS and after DES implantation [11,12]. Neoatherosclerosis was significantly associated with high serum LDL cholesterol level [11], suggesting that the process is similar with that of atherosclerosis progression in the native coronary artery. Furthermore, the fragments of atherosclerotic plaque were often aspirated from the culprit lesions of very late stent thrombosis after BMS implantation, which were histologically indistinguishable from the material aspirated from the culprit lesions of spontaneous acute coronary syndrome in the native coronary arteries [13]. These findings suggest that both very late stent thrombosis and spontaneous acute coronary syndrome are similarly caused by an advanced atherosclerotic lesion with plaque rupture in the majority of cases. Although VLSF is observed both after BMS and after DES implantation, the stented segments are usually stable and rarely cause VLSF up to 5 years after BMS implantation [1]; however, the stented segments often cause VLSF earlier after DES implantation. Cases of acute myocardial infarction occurring at the stented segment 5–10 years after BMS implantation have often been reported previously in many cardiology meetings in Japan, in which coronary angioscopy is commonly detected as a disrupted yellow plaque with thrombus at the culprit lesion that closely resembled with the culprit lesion of acute myocardial infarction in native coronary arteries. Previously, it was thought to be quite natural that the progression of atherosclerosis would lead to the onset of acute coronary syndrome even at the coronary segment where BMS had been implanted previously and was still present inside the vessel wall.
The lesions of in-stent restenosis have been examined by many studies using OCT, IVUS, angioscopy, or near infrared spectroscopy (NIRS) [15–21]. Neoatherosclerosis has been detected more often at the sites of in-stent restenosis after DES implantation than after BMS implantation [17,18], especially in the long term after implantation [15–16,19]. The OCT findings of heterogeneous intima, thin-cap fibroatheroma (TCFA)- like image and intraintima microvessels increased from the early to the very late DES restenotic lesions [19]. The cap thickness was negatively correlated with the follow-up interval [20]. The in-stent restenosis can also be caused by thrombus formation as demonstrated by angioscopy [28,29]. Therefore, the plaque rupture and thrombus formation at the site of advanced neoatherosclerosis would be a major cause of both stent thrombosis and in-stent restenosis in the very late phase of >1 year after implantation both in BMSs and DESs.
We should be careful not to confuse ‘neointima thickening’ with ‘neoatherosclerosis’ after stent implantation. Although the definition of neoatherosclerosis depends on the modality used to define it and the study protocol. In general, tissue with much lipid deposition, for example, yellow plaque by angioscopy, low echoic tissue in IVUS, necrotic or fibrofatty criteria tissue in VH/IB-IVUS, TCFA by OCT and yellow signal in NIRS, with or without some other characteristics of atherosclerotic lesions defined by pathology, for example, microvessels and macrophages, has been regarded as a sign of neoatherosclerosis. The neointima thickening early after BMS implantation is known to be caused by smooth muscle cell hyperplasia with the formation of fibrous tissue without neoatherosclerosis, which would be diseased by atherosclerosis after only 5–10 years. On the other hand, neoatherosclerosis has often been detected at the sites of in-stent restenosis after DES implantation both in early phase and in late-to-very-late phase. Therefore, the correlation between neointima volume and incidence of neoatherosclerosis is a new finding in DESs which has never been detected in BMS.
The neointima evaluated by OCT or IVUS at one year after DES implantation [30,31] had neoatherosclerosis more often in the patients with diabetes mellitus or chronic kidney disease than in those without, suggesting that the risk factors of native coronary atherosclerosis are also the risks of in-stent neoatherosclerosis. Furthermore, the larger neointima volume was associated with the higher incidence of neoatherosclerosis [31,32]. A serial follow-up study at 1 and 2 years after DES implantation by OCT revealed further increase after 1 year in the neointima thickness and in the incidence of lipid-laded neointima and thincap neoatheroma [33]. This suggests that the continuous progression of in-stent atherosclerosis would contribute to the neointima thickening.
Therefore, the formation of the lipid-rich atherosclerotic plaque detected by IVUS or OCT or the formation of yellow plaque detected by angioscopy would be an important common mechanism for the occurrence of stent thrombosis and of restenosis, in other words, VLSF, both after BMS and after DES implantation. This process of neoatherosclerosis would have common mechanisms with the process of atherosclerosis progression in the native coronary artery.
Process of neointima sealing/shielding & atherosclerosis progression after stent implantation detected by intracoronary imaging
At 3–6 months after implantation, BMSs are commonly well covered by white thick neointima [34,35], which is known to be fibrous tissue and is caused mainly by smooth muscle cell hyperplasia. It usually takes 5–10 years for this thick white nonatherosclerotic neointima to become yellow by neoatherosclerosis and to cause the next event of acute coronary syndrome [21,35]. The formation of thick fibrous white neointima over atherosclerotic yellow lesions will play a role of sealing and shielding that makes the vulnerable plaques stable.Once the yellow vulnerable plaque under stent is covered by white thick fibrous neointima, it will take a long time for the formation of vulnerable plaque that is visible from lumen as a yellow plaque in the segment where the BMS has been implanted.
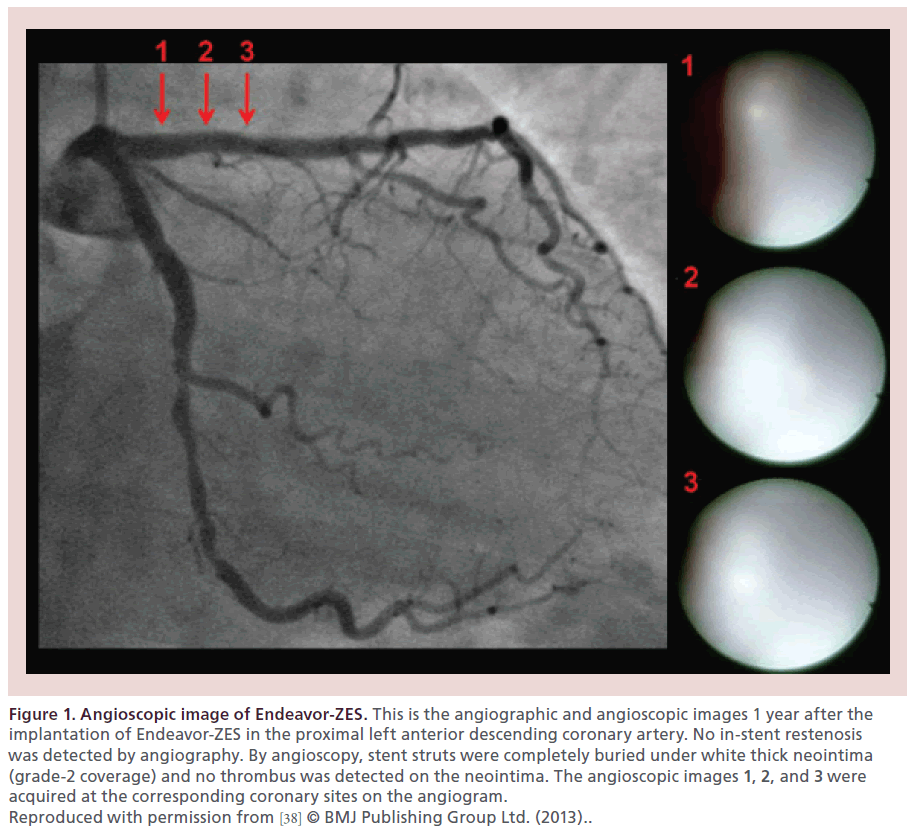
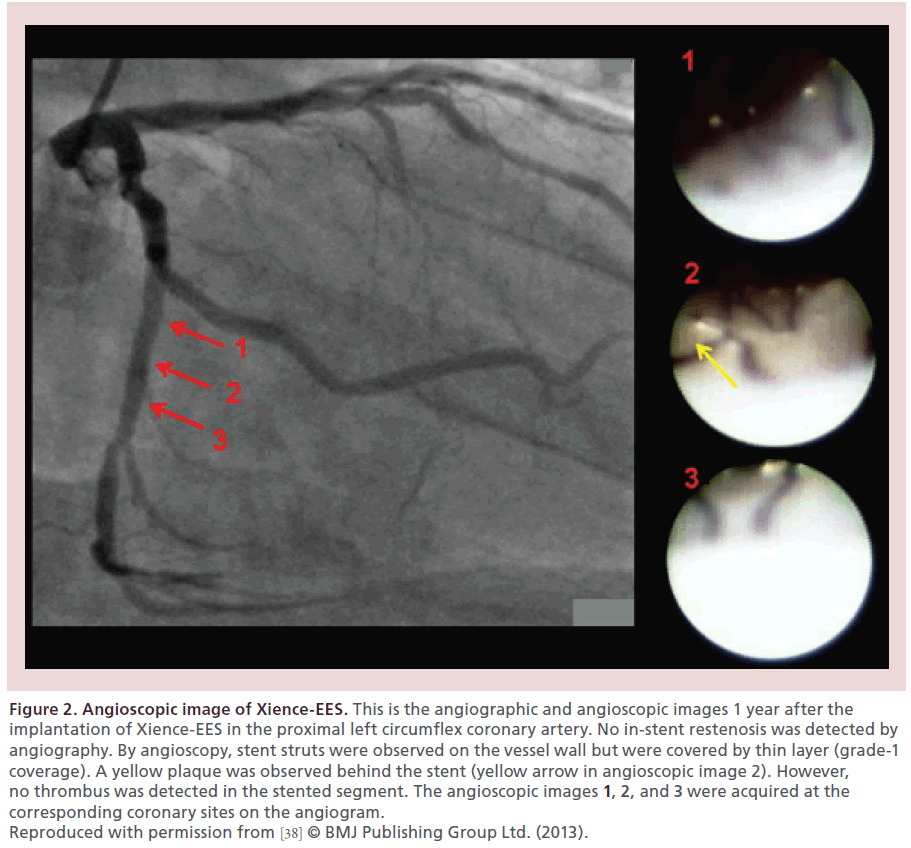
Higo et al. reported for the first time in a living human that the Cypher sirolimus-eluting stent (Cypher-SES, Cordis, NJ, USA) accelerated the formation of yellow plaque at 10 months after implantation [36], which was very uncommon after BMS implantation. A histopathologic study by Nakazawa et al. confirmed the presence of neoatherosclerosis in the neointima after BMS and DES implantation with the shorter implant duration for DESs to develop neoatherosclerosis than for BMSs [9]. The Endeavor zotarolimus-eluting stent (Endeavor-ZES, Medtronic, MN, USA), like BMSs, reduced the yellow color of the stented segment by the formation of white thick neointima [37]. The Xience everolimus-eluting stent (Xience-EES, Abbott Vascular, CA, USA) had very thin neointima and did not change the yellow color of the stented segment [37,38]. Representative angioscopic images of the Endeavor- ZES and of the Xience-EES at 1 year after implantation are presented in Figures 1 & 2, respectively [38].
Figure 1: Angioscopic image of Endeavor-ZES. This is the angiographic and angioscopic images 1 year after the implantation of Endeavor-ZES in the proximal left anterior descending coronary artery. No in-stent restenosis was detected by angiography. By angioscopy, stent struts were completely buried under white thick neointima (grade-2 coverage) and no thrombus was detected on the neointima. The angioscopic images 1, 2, and 3 were acquired at the corresponding coronary sites on the angiogram. Reproduced with permission from [38] © BMJ Publishing Group Ltd. (2013)..
Figure 2: Angioscopic image of Xience-EES. This is the angiographic and angioscopic images 1 year after the implantation of Xience-EES in the proximal left circumflex coronary artery. No in-stent restenosis was detected by angiography. By angioscopy, stent struts were observed on the vessel wall but were covered by thin layer (grade-1 coverage). A yellow plaque was observed behind the stent (yellow arrow in angioscopic image 2). However, no thrombus was detected in the stented segment. The angioscopic images 1, 2, and 3 were acquired at the corresponding coronary sites on the angiogram. Reproduced with permission from [38] © BMJ Publishing Group Ltd. (2013).
The Cypher-SES failed to seal/shield the vulnerable yellow plaque and furthermore accelerated the formation of yellow plaque, probably owing to the inflammation caused by its polymer. Endeavor-ZES and BMS can seal/shield and stabilize the vulnerable yellow plaque under stent. Yellow plaques with large lipid core and thin fibrous-cap, in other words, thin-cap fibroatheroma (TCFA), are regarded as vulnerable plaques that are prone to rupture and cause acute coronary syndrome. On the other hand, thickening of the fibrouscap is thought to play a role of plaque stabilization, which is detected as a reduction of yellow color. Therefore, the formation of thick fibrous white neointima over vulnerable yellow plaques is supposed to play a role of plaque stabilization. The lesion may become vulnerable and yellow again, when the cap becomes thin again due to the progression of atherosclerosis.
Although the Xience-EES has failed to seal/shield the yellow plaque under stent because it forms very thin neointima, it does not accelerate the formation of yellow plaque either. The healing after Xience-EES implantation would be much better than after Cypher- SES implantation and would be similar to that after Endeavor-ZES implantation, because the incidence of thrombus formation at 1 year after implantation was similarly very low in the Xience-EES and Endeavor- ZES in comparison with very high incidence in the Cypher-SES [28,36–39]. Yellow plaque formation could be accelerated by various coronary risk factors [40] and reduced by medical therapy like statins [41,42].Therefore, in combination with these systemic factors and medications, the characteristics of each DES may influence the formation and progression of yellow plaque at the stented segment, which may influence the onset of VLSF later.
Incidence of very-late stent failure observed in clinical trials
According to the results of clinical trials with longterm follow-up up to 5 years available in previous reports or at ClinicalTrials.gov [43], TLR at 1 and 5 years are 4.9% and 9.4% in Cypher-SES [44,45]; 4.4% and 9.1% in Taxus paclitaxel-eluting stent (Taxus- PES, Boston Scientific, MA, USA) [46,47]; 5.9% and 7.5% in Endeavor-ZES [48,49]; and 3.4% and 8.9% in Xience-EES [50]. Therefore, the incidence of VLSF as shown by the yearly TLR between 1 and 5 years is 1.1%/year, 1.2%/year, 0.4%/year and 1.4%/year in Cypher-SES, Taxus-PES, Endeavor-ZES and Xience- EES, respectively, which appears lower in Endeavor- ZES than in other DES.
In the ENDEAVOR III trial, although the angiographic restenosis was higher in Endeavor-ZES than in Cypher-SES at 9 months, cumulative outcomes through 5 years demonstrated that death, cardiac death, and myocardial infarction were significantly lower in Endeavor- ZES than in Cypher-SES [3]. The rate of TLR beyond 9 months was significantly lower in Endeavor-ZES than in Cypher-SES. The superiority of Cypher-SES compared with Endeavor-ZES at 1-year follow-up was also lost after 5 years in the SORT OUT III study [51]. According to the editorial for the latter report [52], close collaboration between physicians, regulatory authorities and device manufacturers is needed for meaningful postmarketing device assessment, and approval of novel coronary devices should be based on the commitment to undertake appropriately designed long-term followup studies. Although Endeavor-ZES was finally demonstrated superior to Cypher-SES after 5 years, Cypher- SES had already been used widely in the world and the Endeavor-ZES had disappeared. Both stents are no longer used in daily practice now, suggesting that the presentation of 5-year follow-up results was too late to influence our daily practice of PCI.
Therefore, we should realize that the incidence of VLSF is very important for the long-term outcome; and 1-year result in the comparison of two stents would not be adequate to determine the better stent. Five-year or longer outcomes should be considered more important than the shorter outcome in those clinical trials. However, we usually cannot wait for the 5-year results; therefore, the evaluation of intracoronary imaging like angioscopy at 1 year may be useful as a surrogate end point to predict long-term clinical outcome.
Association between neoatherosclerosis detected by intracoronary imaging & future event of very-late stent failure
The higher incidence of VLSF in Cypher-SES than in Endeavor-ZES may possibly be explained by the facts that the Cypher-SES accelerates the formation of yellow plaque and the Endeavor-ZES stabilizes it by forming white thick neointima over it. The stability of the stented lesions with the Xience-EES, having no influence on the yellow color of the lesion, may be in between of that of the Endeavor-ZES and that of the Cypher-SES. Therefore, the incidence of VLSF with Xience-EES might be higher than that with Endeavor-ZES, although there is no clinical trial that has compared those two stents directly and the initial outcome at 1 year is far better with Xience-EES than with Endeavor-ZES. Although the TWENTE trial [53] has compared the Xience-EES and Resolute- ZES (Medtronic, MN, USA), the Resolute-ZES and Endeavor-ZES appear completely different from the angioscopic viewpoint. Regarding the angioscopic image at 1 year after implantation, Resolute-ZES resembles Xience-EES, while Endeavor-ZES resembles a BMS. Therefore, we cannot speculate the results of Endeavor-ZES from those of Resolute-ZES.
According to the accumulated findings so far, we believe that the extent of atherosclerosis in the DESimplanted segment as shown by its yellow color would be associated with a future event of VLSF. We have started a single-center prospective observational study (DESNOTE study: Detect the Event of very late Stent failure from the drug-eluting stent NOT well covered by nEointima determined by angioscopy) to demonstrate that the angioscopic findings, especially the presence of yellow plaque, at 1 year after DES implantation would be a risk of future VLSF. All patients with successful angioscopic examination at planned 1-year follow-up after the implantation of a DES in the native coronary artery, without any event of stent failure before the follow-up, have been enrolled since July 2004 and have been clinically followed up for the occurrence of VLSF at our outpatient clinic. Yellow color, neointima coverage and thrombus at the site of DES implantation are examined by angioscopy. VLSF is defined as cardiac death, myocardial infarction or unstable angina at the target stent, or TLR at the target stent. Cardiac death is defined as the death without known noncardiac cause.
A report from the CREDO-Kyoto Registry demonstrated that statin use was associated with the lower risk of late TLR after Cypher-SES implantation [54], suggesting that prevention of atherosclerosis progression by statin therapy can reduce the incidence of VLSF. Statin therapy is known to prevent or regress coronary atherosclerosis and to reduce the yellow color of the plaques by the thickening of the fibrous cap, and thus, this finding of CREDO-Kyoto Registry supports our hypothesis that yellow plaque formation would be an important mechanism involved in the occurrence of VLSF.
The issue of neoatherosclerosis was a bigger problem with the 1st generation DESs that accelerated atherosclerosis progression probably through increased inflammation than with the newer DESs as the incidence of VLSF was higher in the former in comparison with the latter. However, VLSF still exists and is still an important problem with the newer DESs. It is quite natural that the process of atherosclerosis progression goes on even after the implantation of stent in the coronary arteries. First generation DESs accelerated it and BMS stabilized it by forming a thick fibrous cap on vulnerable plaques; however, the newer DESs appear to have no significant influence on the natural process of atherosclerosis progression. In other words, the next event of VLSF at the site of new DES implantation would be caused by the natural process of atherosclerosis progression. Therefore, the early detection of neoatherosclerosis or in-stent atherosclerosis progression and its prevention or regression would be beneficial to improve long-term outcomes of patients with DES implantation [23].
Conclusion
One of the probable mechanisms of VLSF is the progression of atherosclerosis. In-stent atherosclerosis evaluated by the presence of yellow plaque at one year after implantation of DES may be a risk of VLSF. In addition to statin therapy, general approach to prevent atherosclerosis may be useful to prevent VLSF.
Future perspective
In the clinical trials that compare different stents, 1-year results of intracoronary imaging might be useful to predict the long-term clinical outcome. To prevent or reduce the incidence of VLSF, aggressive statin therapy may become a recommended therapy rather than an antithrombotic therapy as in the prevention of acute coronary syndrome.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Very-late stent failure evaluated by intracoronary imaging
• Very-late stent failure is strongly associated with, and probably caused mainly by, atherosclerosis progression presented as yellow plaque formation by angioscopy.
Atherosclerosis progression and neointima sealing/shielding after stent implantation
• A bare metal stent (BMS) with yellow vulnerable plaque under stent is usually covered by a thick fibrous white neointima, which stabilizes the lesion.
• Drug-eluting stents (DESs), excluding the Endeavor-ZES, form very thin neointima and cannot seal/shield the vulnerable plaque under stent.
• The Cypher-SES accelerates the formation of vulnerable yellow plaque.
Very-late stent failure in clinical trials
• The incidence of very-late stent failure (VLSF) appears lower in the Endeavor-ZES than in other DESs, although there is no adequate evidence from direct comparisons.
Association between yellow plaque and future event of VLSF
• The clinical trial that examines the association between intracoronary imaging (angioscopy) at one year and long-term clinical outcome is now under way. We believe that the extent of atherosclerosis in the DESimplanted segment as shown by its yellow color would be associated with the future event of VLSF.
References
- Natsuaki M, Morimoto T, Furukawa Y et al. Late adverse events after implantation of sirolimus-eluting stent and bare-metal stent: long-term (5-7 years) follow-up of the Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2. Circ. Cardiovasc. Interv. 7(2), 168–179 (2014).
- Eisenstein EL, Wijns W, Fajadet J et al. Long-term clinical and economic analysis of the Endeavor drug-eluting stent versus the Driver bare-metal stent: 4-year results from the ENDEAVOR II trial (Randomized Controlled Trial to Evaluate the Safety and Efficacy of the Medtronic AVE ABT-578 Eluting Driver Coronary Stent in de novo Native Coronary Artery Lesions). JACC Cardiovasc. Interv. 2(12), 1178–1187 (2009).
- Kandzari DE, Mauri L, Popma JJ et al. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents. 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De novo Native Coronary Artery Lesions). JACC Cardiovasc. Interv. 4(5), 543–550 (2011).
- Leon MB, Kandzari DE, Eisenstein EL et al. Late safety, efficacy, and cost-effectiveness of a zotarolimus-eluting stent compared with a paclitaxel-eluting stent in patients with de novo coronary lesions: 2-year follow-up from the ENDEAVOR IV trial (Randomized, Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System versus the Taxus Paclitaxel-Eluting Coronary Stent System in de novo Native Coronary Artery Lesions).JACC Cardiovasc. Interv. 2(12), 1208–1218 (2009).
- Garg S, Serruys P, Onuma Y et al. 3-year clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: the SPIRIT II trial (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions). JACC Cardiovasc. Interv. 2(12), 1190–1198 (2009).
- Stone GW, Midei M, Newman W et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation 119(5), 680–686 (2009).
- Stone GW, Simonton CA, Sood P, Kereiakes DJ. SPIRIT IV Investigators. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J. Am. Coll. Cardiol. 58(1), 19–25 (2011).
- Onuma Y, Miquel-Hebert K, Serruys PW. SPIRIT II Investigators. Five-year long-term clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery disease: the SPIRIT II trial. EuroIntervention 8(9), 1047–1051 (2013).
- Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovasc. Imaging 2(5), 625–628 (2009).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18), 2435– 2441 (2007).
- Amabile N, Souteyrand G, Ghostine S et al. Very late stent thrombosis related to incomplete neointimal c verage r neoatherosclerotic plaque rupture identified by ptical coherence tomography imaging. Eur. Heart J. Cardi vasc. Imaging 15(1), 24–31 (2014).
- Kang SJ, Lee CW, Song H et al. OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc. Imaging 6(6), 695–703 (2013).
- Yamaji K, Inoue K, Nakahashi T et al. Bare metal stent thrombosis and in-stent neoatherosclerosis. Circ. Cardiovasc. Interv. 5(1), 47–54 (2012).
- Liistro F, Stankovic G, Di Mario C et al. First clinical experience with a paclitaxel derivate-eluting polymer stent system implantation for in-stent restenosis: immediate and long-term clinical and angiographic outcome. Circulation 105(16), 1883–1886 (2002).
- Araki T, Nakamura M, Sugi K. Characterization of in-stent neointimal tissue components following drug-eluting stent implantation according to the phase of restenosis using a 40-MHz intravascular ultrasound imaging system. J. Cardiol. 64(6), 423–429 (2014).
- Lee SY, Shin DH, Mintz GS et al. Optical coherence tomography-based evaluation of in-stent neoatherosclerosis in lesions with more than 50% neointimal cross-sectional area stenosis. EuroIntervention 9(8), 945–951 (2013).
- Ali ZA, Roleder T, Narula J et al. Increased thin-cap neoatheroma and periprocedural myocardial infarction in drug-eluting stent restenosis: multimodality intravascular imaging of drug-eluting and bare-metal stents. Circ. Cardiovasc. Interv. 6(5), 507–517 (2013).
- Ando H, Amano T, Takashima H et al. Differences in tissue characterization of restenotic neointima between sirolimus-eluting stent and bare-metal stent: integrated backscatter intravascular ultrasound analysis for in-stent restenosis. Eur. Heart J. Cardiovasc. Imaging 14(10), 996–1001 (2013).
- Habara M, Terashima M, Nasu K et al. Morphological differences of tissue characteristics between early, late, and very late restenosis lesions after first generation drug-eluting stent implantation: an optical coherence tomography study.Eur. Heart J. Cardiovasc. Imaging 14(3), 276 –284 (2013).
- Kang SJ, Mintz GS, Akasaka T et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation 123(25), 2954–2963 (2011).
- Yokoyama S, Takano M, Yamamoto M et al. Extended follow-up by serial angioscopic observation for bare-metal stents in native coronary arteries: from healing response to atherosclerotic transformation of neointima. Circ. Cardiovasc. Interv. 2(3), 205–212 (2009).
- Nakazawa G. Stent thrombosis of drug eluting stent: pathological perspective. J. Cardiol. 58(2), 84–91 (2011).
- Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J. Am. Coll. Cardiol. 59(23), 2051–2057 (2012).
- Ueda Y, Ohtani T, Shimizu M, Hirayama A, Kodama K. Assessment of plaque vulnerability by angioscopic classification of plaque color. Am. Heart J. 148(2), 333–335(2004).
- Ueda Y, Asakura M, Yamaguchi O, Hirayama A, Hori M, Kodama K. The healing process of infarct-related plaques. Insights from 18 months of serial angioscopic follow-up.J. Am. Coll. Cardiol. 38(7), 1916–1922 (2001).
- Hasegawa K, Tamai H, Kyo E et al. Histopathological findings of new in-stent lesions developed beyond five years.Catheter. Cardiovasc. Interv. 68(4), 554–558 (2006).
- Matsuo K, Ueda Y, Nishio M et al. Very late stent thrombosis at 2.5 years after sirolimus-eluting stent implantation with prior angioscopic image of culprit lesion:a case report.J. Cardiology Cases 5(1), e12–e15 (2012).
- Ueda Y, Kodama K. Chapter 39. In: What next? Coronary Stent Restenosis. Tintoiu IC, Popma JJ, Bae JH, Rivard A,Galassi AR, Cristian G (Eds). The Publishing House of the Romanian Academy, Bucharest, Romania, 609–616 (2011).
- Oikawa Y, Yajima J, Costa MA et al. Intravascular ultrasound, angioscopic and histopathological characterisation of heterogeneous patterns of restenosis after sirolimus-eluting stent implantation: insights into potential “thromborestenosis” phenomenon. EuroIntervention 6(3), 380–387 (2010).
- Tian F, Chen Y, Liu H, Zhang T, Guo J, Jin Q. Assessment of characteristics of neointimal hyperplasia after drug-eluting stent implantation in patients with diabetes mellitus: an optical coherence tomography analysis. Cardiology 128(1), 34–40 (2014).
- Hong YJ, Jeong MH, Choi YH et al. Relation between renal function and neointimal tissue characteristics after drug-eluting stent implantation: virtual histology-intravascular ultrasound analysis. J. Cardiol. 64(2), 98–104 (2014).
- Vergallo R, Yonetsu T, Uemura S et al. Correlation between degree of neointimal hyperplasia and incidence and characteristics of neoatherosclerosis as assessed by optical coherence tomography. Am. J. Cardiol. 112(9), 1315–1321 (2013).
- Kim JS, Hong MK, Shin DH et al. Quantitative and qualitative changes in DES-related neointimal tissue based on serial OCT. JACC Cardiovasc. Imaging 5(11), 1147–1155 (2012).
- Ueda Y, Nanto S, Komamura K, Kodama K. Neointimal coverage of stents in human coronary arteries observed by angioscopy. J. Am. Coll. Cardiol. 23(2), 341–346 (1994).
- Asakura M, Ueda Y, Nanto S et al. Remodeling of in-stent neointima, which became thinner and transparent over 3 years. Serial angioscopic and angiographic follow-up. Circulation 97(20), 2003–2006 (1998).
- Higo T, Ueda Y, Oyabu J et al. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovasc. Imaging 2(5), 616–624 (2009).
- Akazawa Y, Matsuo K, Ueda Y et al. Atherosclerotic change at one year after implantation of Endeavor zotarolimus - eluting stent vs. everolimus-eluting stent. Circ. J. 78(6), 1428–1436 (2014).
- Matsuo K, Ueda Y, Nishio M et al. A higher colour g ade yellow plaque was detected at ne year after implantation of an everolimus-eluting stent than after a z ta limus-eluting stent. Heart Asia 5, 192–196 (2013).
- Oyabu J, Ueda Y, Ogasawara N, Okada K, Hirayama A, Kodama K. Angioscopic evaluati n f ne intima c verage: sirolimus drug-elu ing s ent versus bare metal stent. Am. Heart J. 152(6), 1168–1174 (2006).
- Kurihara O, Takano M, Yamamoto M et al. Impact of prediabetic s a s on coronary a herosclerosis: a multivessel angioscopic st dy. Diabetes Care 36(3), 729–733 (2013).
- Hirayama A, Saito S, Ueda Y et al. Qualitative and quantitative changes in coronary plaque associated with atorvastatin therapy. Circ. J. 73(4), 718–725 (2009).
- Kodama K, Komatsu S, Ueda Y et al. Stabilization and regression of coronary plaques treated with pitavastatin proven by angioscopy and intravascular ultrasound–the TOGETHAR trial. Circ. J. 74(9), 1922–1928 (2010).
- ClinicalTrials.Gov https://clinicaltrials.gov
- Holmes DR Jr, Leon MB, Moses JW et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 109(5),634–640 (2004).
- Weisz G, Leon MB, Holmes DR Jr et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in de-novo Native Coronary Lesions) Trial. J. Am. Coll. Cardiol. 53(17), 1488–1497 (2009).
- Stone GW, Ellis SG, Cox DA et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation 109(16), 1942–1947 (2004).
- Ellis SG, Stone GW, Cox DA et al. Long-term safety and efficacy with paclitaxel - eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV - SR: Treatment of De novo Coronary Disease Using a Single Paclitaxel-ElutingStent). JACC Cardi vasc. Interv. 2(12), 1248–1259 (2009).
- Fajadet J, Wijns W, Laarman GJ et al. Randomized, double- blind, multicenter study f the Endeavor zotarolimus-eluting phsphrylchline-encapsulated stent for treatment of native coronary artery lesi ns: clinical and angiographic results of the ENDEAVOR II Trial. Circulation 114(8), 798–806 (2006).
- Fajadet J, Wijns W, Laarman GJ et al. Randomized, doubleblind,multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II Trial. Circulation 114(8), 798–806 (2006).
- Stone GW, Midei M, Newman W et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA 299(16), 1903–1913 (2008).
- Maeng M, Tilsted HH, Jensen LO et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III Study): a multicentre, open-label, randomised superiority trial. Lancet 383(9934), 2047–2056 (2014).
- Stefanini GG, Windecker S. Stent performance: never too late to sort it out. Lancet 383(9934), 2024–2026 (2014).
- Löwik MM, Lam MK, Sen H et al. Safety of second-generation drug-eluting stents three years after randomised use in the TWENTE trial. EuroIntervention doi: 10.4244/ EIJY14M08_11 (2014) (Epub ahead of print).
- Natsuaki M, Nakagawa Y, Morimoto T et al. Impact of statin therapy on late target lesion revascularization after sirolimus-eluting stent implantation (from the CREDO-Kyoto Registry Cohort-2). Am. J. Cardiol. 109(10), 1387–1396 (2012).