Perspective - Interventional Cardiology (2010) Volume 2, Issue 2
Drug-eluting stents in patients on long-term oral anticoagulation therapy: a mission impossible?
- Corresponding Author:
- KE Juhani Airaksinen
Department of Medicine
Cardiology Unit, Turku University Hospital
Kiinamyllynkatu 4–8, 20520 Turku, Finland
Tel: +358 2 313 1005
Fax: +358 2 313 2030
E-mail: juhani.airaksinen@tyks.fi
Abstract
Keywords
antithrombotic treatment, bleeding, clopidogrel, coronary artery stent, warfarin
Percutaneous coronary intervention (PCI) with drug-eluting stents (DES) has markedly reduced restenosis and the subsequent need for repeat revascularization procedures and has become common practice [1,2]. However, the risk of late stent thrombosis with these devices has led to the recommendation of prolonged dual antiplatelet therapy (DAT) [3,4].
It is estimated that 5% of patients undergoing PCI are on long-term oral anticoagulation (OAC) therapy owing to underlying chronic medical conditions such as atrial fibrillation (AF) or mechanical heart valve. This chronic OAC patient group presents unique challenges in navigating the delicate balance of preventing ischemic events with DAT, while mitigating the risk of stroke and other embolic events with OAC, without a resultant unacceptably high bleeding risk [5,6]. Stent selection and concomitant antithrombotic strategies are key considerations when finding the best balance between these opposite threats.
In this article, we first focus on the available reports on current stenting practice in OACtreated patients and continue with an analysis of the available evidence on the magnitude of the competing risks of restenosis, stent thrombosis, stroke and bleeding events in this complex patient population. Last, we present practical suggestions for the treatment of this growing patient population at high risk of bleeding events.
Previous studies on the use of DES in patients on OAC
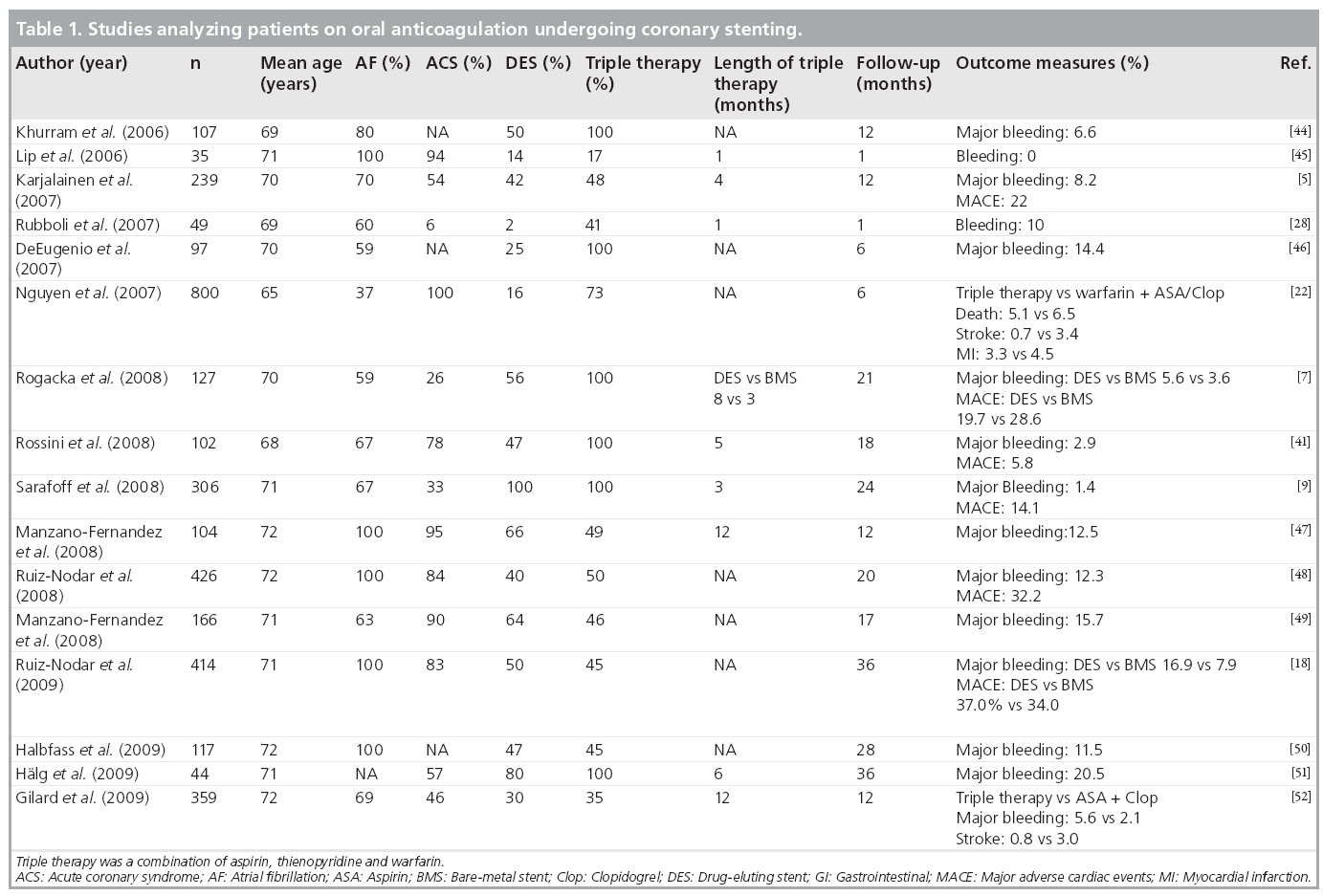
We conducted literature searches in PubMed/ MEDLINE and the Cochrane Library (including the Cochrane Database of Systematic Reviews and the Cochrane Controlled Trials Registry) on English language articles and found 16 studies describing outcomes in OAC patients undergoing coronary stenting and providing information on stent use (Table 1). Most publications reported retrospective analyses of single-center consecutive patient series undergoing PCI in different settings. No randomized trials were available. The data are heterogeneous, and the reporting of clinical parameters associated with thrombotic or bleeding events are even more so.
The use of DES ranged from 2 to 100% between the reports and centers. Only two of the trials presented data on comparison of DES versus bare-metal stents (BMS) (Table 1) [7,8]. Rogacka et al. showed no significant difference between DES or BMS with respect to major bleeding or major adverse cardiac events (MACE) [7], whereas Ruiz-Nodar et al. concluded that the routine use of DES in this patient population does not appear to be justified on the basis of adverse outcome in the DES-treated patients [8]. At the other end of the spectrum, Sarafoff et al. demonstrated that the use of DES was feasible and safe in 515 patients receiving either a triple therapy of OAC plus aspirin and clopidogrel or DAT [9]. In this trial, the choice of DAT or triple therapy with a low target international normalized ratio (INR) of 2.0–2.5 was made on the basis of an individual assessment of thromboembolic risk in each patient.
Data on antithrombotic therapy after stenting was variable and no data on bleeding risk assessment were reported. Generally, major bleeding with triple therapy increased by 3.2–6.6-fold compared with DAT alone. The incidence of stroke and stent thrombosis was rarely reported, but when it was, it was lowest with the triple therapy. One study argued against the use of DAT in stroke prevention and also reported a high incidence of stent thrombosis with the combination OAC and aspirin [5].
Risk of restenosis & stent thrombosis
Percutaneous coronary intervention with stenting compared with balloon angioplasty alone has markedly reduced the rates of restenosis. At present, the incidence of clinically driven restenosis is approximately 3–10% with BMS and 2–5% with DES in large registry studies, varying according to the lesion and patient characteristics [1,2,10,11].
The well-known downside of stenting is the risk of stent thrombosis, which has received special attention due to the high mortality and morbidity of the complication. With the current antithrombotic strategies, most (~1%) stent thromboses occur early (<30 days), but late thrombosis is reported to occur at an annual rate of approximately 0.6% up to 4 years after DES implantation [12,13]. A higher rate of late stent thrombosis has been observed after acute coronary syndrome than in stable patients in post-mortem analysis of patients who died after DES implantation [14]. Premature DAT discontinuation has been the most important predisposing factor for stent thrombosis [15]. Recent data has suggested that patient-related factors such as age, hypertension, diabetes, smoking, renal failure, low ejection fraction and female gender are independently associated with stent thrombosis [16].
Four early randomized trials showed that DAT cannot be safely replaced by a warfarin plus aspirin combination in preventing stent thrombosis [17]. The recommended duration of DAT is at least 1 month in patients receiving BMS, 3 months in patients receiving DES from limus family and 6 months of aspirin and clopidogrel in patients receiving paclitaxel-eluting DES. DAT has been proven to be beneficial in patients with both non-ST-elevation myocardial infarction [18] and ST-elevation myocardial infarction [19], and should be maintained for up to 12 months in these indications. The major problem with the use of DES is that premature stopping of the longer clopidogrel treatment may cause a tenfold increased risk of stent thrombosis [15].
Risk of stroke
Devastating, irreversible consequences of stroke have been self-evident for the clinicians. AF is the most common risk factor for stroke, increasing the incidence of embolic stroke from 1% to over 10% per year depending on concomitant risk factors. In addition to AF, other conditions may put patients at a high risk of thromboembolic complications and, for example, patients with mechanical valve prosthesis confer an annual risk of 10–91% depending on the position and type of prosthesis and concomitant risk factors [20].
Oral anticoagulation reduces the risk of stroke by two-thirds, as demonstrated by well-designed clinical trials for the primary and secondary prevention of stroke and thromboembolism in a wide spectrum of clinical conditions. The greatest benefit is seen in those people who are in the high-risk category for having a stroke. The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE-W) trial showed that DAT cannot replace OAC in stroke prevention in patients with AF [21], and recent observational studies on clinical practice also support this conclusion in patients on home warfarin undergoing PCI [5,8,22].
Risk of bleeding complications
The annual risk of major bleedings among ‘real world’ patients on OAC is estimated to be approximately 3%. The bleeding risk seems to be even higher in the first year of treatment and in the elderly population [23]. Adding aspirin to warfarin therapy confers a 1–2% absolute increase in major bleeding per year compared with warfarin alone [24]. In a recent Danish study, triple therapy of OAC, aspirin and clopidogrel was associated with a threefold increase in bleeding complications compared with OAC alone [25]. Strikingly, the bleeding risk of clopidogrel plus OAC, which has been recommended as a potentially safer combination than triple therapy, was only slightly lower than the combination of all three drugs.
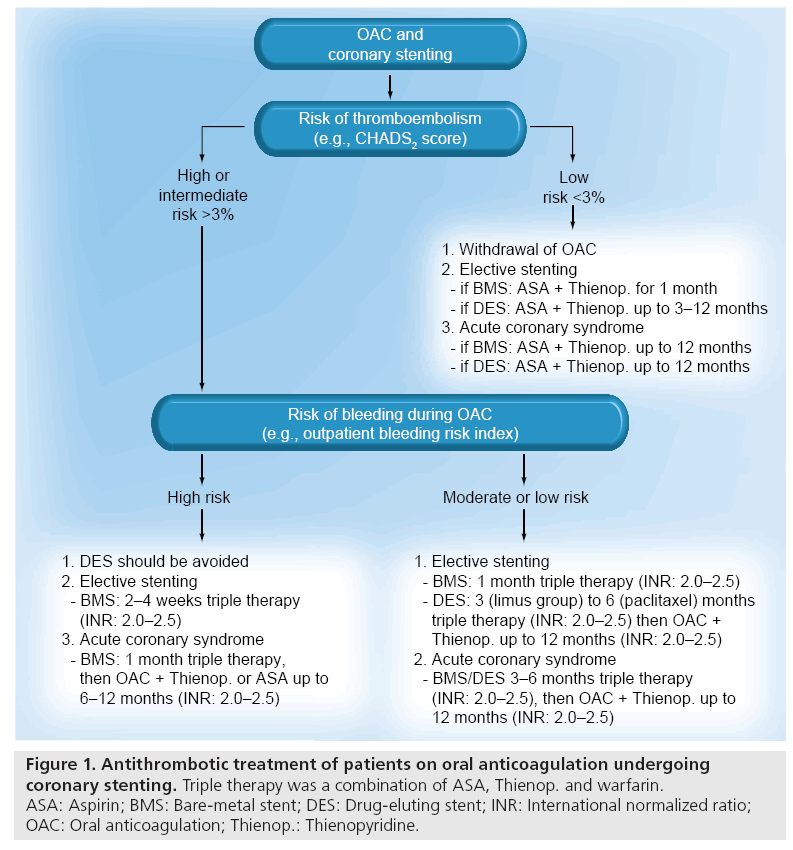
Bleeding complications are the most frequent nonischemic complications of PCI, especially in the treatment of acute coronary syndromes. It is estimated that the annual frequency of major bleeding ranges from 2 to 15% across the spectrum of PCI and greatly depends on the type of antithrombotic treatment and use of invasive procedures. Most of the bleeding events occur early during the hospital phase. In the large CRUSADE Registry (an ongoing, voluntary, observational data collection and quality improvement initiative), the incidence of in-hospital major bleeding events was as high as 9.4% [26]. The incidence of bleeding events seems to be even higher when these patients are on long-term OAC and need concomitant potent antiplatelet agents owing to PCI [6,27]. Triple therapy is the most frequently used drug regimen in this scenario. The downside of triple therapy is the high incidence of bleeding complications, ranging up to 21% in small single-center registries (Table 1). According to a pooled analysis, the incidence of major bleeding increased from 4.6 to 10.3% when the treatment period increased from 1 month to 6–12 months or more [28]. On the basis of these considerations, the duration of triple therapy is critical for the bleeding events and should be minimized after individual assessment of risk for ischemic, thromboembolic and bleeding events resulting from available treatment choices (Figure 1).
Figure 1: Antithrombotic treatment of patients on oral anticoagulation undergoing coronary stenting. Triple therapy was a combination of ASA, Thienop. and warfarin. ASA: Aspirin; BMS: Bare-metal stent; DES: Drug-eluting stent; INR: International normalized ratio; OAC: Oral anticoagulation; Thienop.: Thienopyridine.
Assessment of thromboembolic & bleeding risk
The risk factors and consequences of restenosis and stent thrombosis are well known for the interventional cardiologists from the everyday practice. The CHADS2 score successfully stratifies AF patients according to risk of stroke [29]. CHADS2 is measured using five common risk factors: heart failure, hypertension, age over 75 years, diabetes and previous stroke/transient ischemic attack (1 point for each risk factor except 2 points for stroke). A score of 4–6 identifies patients at high risk, a score of 2–3 an intermediate risk, and a score of 0–1 indicates low-risk patients. Other conditions may also put patients at a high risk of stroke, for example, patients with a mechanical valve prosthesis confer a very high risk of thromboembolic complications depending on the position and type of prosthesis and concomitant risk factors if they are not treated with OAC [20].
The importance of avoiding bleeding complications has become more evident, since they have turned out to be highly predictive of mortality across a broad spectrum of patients undergoing PCI [30–32]. The best documented bleeding risk factors in patients using OAC include old age, high blood pressure and history of bleeding or cerebrovascular disease. Anemia, renal failure, female gender, recent myocardial infarction and simultaneous use of antiplatelet therapy also appear to have predictive value, at least in certain clinical situations. There are four published bleeding risk scores that have been validated for bleeding risk in patients on OAC [33]. Of these models, the most often used is the outpatient bleeding risk index, which was developed based on a study that identified independent risk factors for major bleeding, including history of stroke, age over 65 years, history of GI tract bleeding, and the presence of one or more comorbid conditions (recent myocardial infarction, renal insufficiency, severe anemia or diabetes). Based on this classification, the patient is considered to be at a low (zero risk factors), moderate (one or two risk factors) or high risk (three or four risk factors) for bleeding. This index has been validated prospectively and demonstrated to reach acceptable discrimination among the risk categories. In the original report, it was found that the cumulative rates for major bleeding at 1 year varied from 3 to 12 to 48% according to the risk category [34,35].
How to avoid bleeding complications?
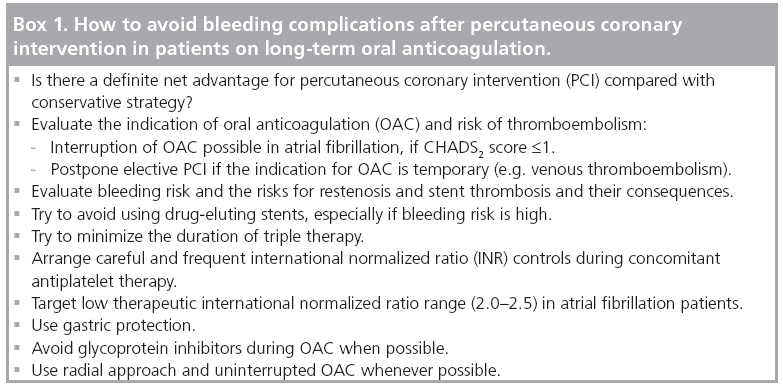
Evaluation of patient’s propensity to ischemic, thromboembolic and bleeding complications is the basis of all individual treatment decisions (Figure 1 & Box 1). If there is a clear indication for OAC, triple therapy is the current recommendation for patients undergoing PCI [36–39]. Since the duration of triple therapy seems to be crucial in the prevention of bleeding events, it should be minimized by limiting DES to those clinical and/or anatomical situations where a significant net benefit is expected as compared with BMS. This pre-requisite leaves little space for the use of DES, especially in patients with clinical bleeding risk factors (as described previously). When using BMS in stable coronary artery disease, the recommended duration of triple therapy is 2–4 weeks according to the individual risks of bleeding complications, followed by longterm OAC therapy or a combination of OAC plus clopidogrel or aspirin (up to 12 months) in patients with a lower bleeding risk.
Optimal duration of triple therapy is still a question of debate in acute coronary syndromes and when using DES, but the general recommendation is to continue treatment up to 12 months [6,39]. However, it seems that the risk of stent thrombosis declines more rapidly than the risk of bleeding complications, rendering the net outcome unfavorable with lengthy use of triple therapy when the patient is at high risk of bleeding [5]. In selected patients at high risk for bleeds, triple therapy may be replaced by a combination of clopidogrel and OAC [5,22], although its safety relative to triple therapy has been questioned.
The risk of bleeding during OAC is related to the intensity of anticoagulation [40]. Thus, it is reasonable to adjust the OAC intensity and target to a lower therapeutic range of INR (2.0–2.5).
This strategy has been shown to lead to a low incidence of bleeding complications in patients on triple therapy after PCI, without compromising the efficacy against stroke and ischemic complications (Table 1) [9,41]. Furthermore, wide fluctuations and overshoots in INR are known to predispose to bleeding complications, underscoring the importance of frequent INR controls preferably in dedicated OAC clinics. If a patient belongs to the lowrisk category (CHADS2 score ≤1), the indication for OAC is relative, and it can usually (at least temporarily) be replaced by DAT (1 month after BMS and up to 12 months after DES). Gastric protection with proton-pump inhibitors is considered useful during triple therapy in spite of the potential attenuation of clopidogrel effects, at least with omeprazole [42,43]. Major bleeding events should be treated aggressively, but inadvertent stopping of antihrombotic treatment owing to minor bleeding events is not wise.
Conclusion
Current guidelines and expert opinions recommend that DES should be avoided or strictly limited to those situations where a significant benefit is expected as compared with BMS [27,44]. Triple therapy is recommended for the prevention of stent thrombosis, but its duration should be individualized according to the stent type and bleeding risk of the patient. These recommendations are largely based on limited evidence obtained from small, single-center and retrospectively analyzed cohorts. Consequently, there is a definite need for largescale registries and prospective clinical studies to determine the optimal management of patients on home OAC undergoing coronary interventions. A continuous focus on educating physicians to tailor antithrombotic therapy according to the patient’s risk profile is also needed.
Future perspective
All these recommendations are based on weak evidence obtained from small, single-center and retrospectively analyzed cohorts; the present practice in this field is highly variable and appears to be based on local opinions as shown by Table 1. Thus, there is a definite need for large-scale registries and prospective clinical studies assessing the optimal management of patients with a concomitant need for OAC who are undergoing coronary stenting. Until then, debate over the optimal management strategy of this increasing patient group is likely to continue. The availability of new drugs (dabigatran, prasugrel and ticagrelor), new-generation DES and bioactive stents may further complicate treatment decisions, since data on their performance in this patient population are lacking.
A prospective, multicenter registry – Management of Patients with Atrial Fibrillation Undergoing Coronary Artery Stenting (AFCAS) – aiming at prospectively evaluating antithrombotic and stenting strategies has been launched in several European countries [101]. The first results of this study will hopefully contribute to shedding some light on this common issue in early 2010. Another registry sponsored by the Working Group on Thrombosis – the Real Life Antithrombotic Stent Evaluation Registry (LASER) [102] – has just begun.
The Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation (ISAR-TRIPLE) trial will provide an answer to the hypothesis that reducing the duration of clopidogrel therapy from 6 months to 6 weeks after DES implantation is associated with improved clinical outcomes in patients on aspirin and an oral anticoagulant [103]. The What is the Optimal Antiplatelet and Anticoagulant in Patients With Oral Anticoagulation and Stenting (WOEST) study [104] will assess the hypothesis that the combination of warfarin and clopidogrel 75 mg/day is superior to triple therapy (warfarin + clopidogrel 75mg/day + aspirin 80 mg/day) with respect to bleeding complications, while equally safe with respect to the prevention of thrombotic complications in patients with both indications for warfarin use and DAT (clopidogrel 75 mg/day + aspirin 80 mg/day). These trials are expected to run until 2011–2012.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Executive summary
Current practice
▪ Approximately 5% of patients referred for coronary stenting are on long-term oral anticoagulation.
▪ Use of drug-eluting stents in this patient group varies from 2 to 100% according to local practice.
▪ Geographical and interhospital variation in the use of antithrombotic treatments is wide.
▪ Triple therapy with oral anticoagulation plus aspirin and clopidogrel is the most often used initial therapy.
Risks of restenosis, stent thrombosis, stroke & major bleeding
▪ Risks of restenosis and stent thrombosis do not appear to be abnormally high.
▪ Annual risk of stroke ranges from 1 to 90% depending on the underlying conditions.
▪ Continuous oral anticoagulation is the cornerstone of stroke prevention.
▪ Bleeding events are the major preventable problem in this fragile patient group.
How to avoid excessive bleeding risk
▪ Evaluate the indications for percutaneous coronary intervention and oral anticoagulation.
▪ Evaluate the bleeding and stroke risk of the individual patient.
▪ Use drug-eluting stents only if they have significant net advantage over bare-metal stents.
▪ Minimize the duration of triple therapy
▪ Target international normalized ratio to the low therapeutic level of 2.0–2.5.
▪ Arrange careful and frequent international normalized ratio controls.
▪ Use a radial approach and uninterrupted oral anticoagulation if possible.
▪ Avoid glycoprotein inhibitors when possible.
▪ Use gastric protection.
References
Papers of special note have been highlighted as:
▪ of interest
- Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ: A hierarchical bayesian meta-analysis of randomized clinical trials of drug-eluting stents. Lancet 364, 583–591 (2004).
- Stone GW, Moses JW, Ellis SG et al.: Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N. Engl. J. Med. 356, 998–1008 (2007).
- Bavry AA, Kumbhani DJ, Helton TJ et al.: What is the risk of stent thrombosis associated with the use of paclitaxel-eluting stents for percutaneous coronary intervention? J. Am. Coll. Cardiol. 45, 941–946 (2005).
- Iakovou I, Schmidt T, Bonizzoni E et al.: Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- Karjalainen PP, Porela P, Ylitalo A et al.: Safety and efficacy of combined antiplateletwarfarin therapy after coronary stenting. Eur. Heart J. 28, 726–732 (2007).
- Rubboli A, Halperin JL, Airaksinen KE et al.: Antithrombotic therapy in patients treated with oral anticoagulation undergoing coronary artery stenting. An expert consensus document with focus on atrial fibrillation. Ann. Med. 40, 428–436 (2008).
- Rogacka R, Chieffo A, Michev I et al.: Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral antiocoagulation. J. Am. Coll. Cardiol. Interv. 1, 56–61 (2008).
- Ruiz-Nodar JM, Marin F, Sanchez-Paya J et al.: Efficay and safety of drug-eluting stent use in patients with atrial fibrillation. Eur. Heart. J. 30, 932–939 (2009).
- Sarafoff N, Drepepa G, Mehilli J et al.: Aspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulation. J. Intern. Med. 264, 472–480 (2008).
- James SK, Stenestrand U, Lindbäck J et al.: Long-term safety and efficacy of drug-eluting versus bare-metal stents in Sweden. N. Engl. J. Med. 360, 1933–1945 (2009).
- Moreno R, Martin-Reyes R, Jimenez-Valero S et al.: Determining clinical benefits of drug-eluting coronary stents according to the population risk profile: a meta-regression from 31 randomized trials. Int. J. Cardiol. (2009). DOI:10.1016/j.ijcard.2009.10.014 (Epub ahead of print).
- Wenaweser P, Daemen J, Zwahlen M et al.: Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. J. Am. Coll. Cardiol. 52, 1134–1140 (2008).
- Daemen J, Wenaweser P, Tsuchida K et al.: Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369, 667–678 (2007).
- Nakazawa G, Finn AV, Joner M et al.: Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients. Circulation 118, 1138–1145 (2008).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al.: Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents. An observational study of drug-eluting versus bare-metal stents (BASKET-LATE). J. Am. Coll. Cardiol. 48, 2584–2591 (2006).
- Daemen J, Serruys PW: Drug-eluting stent update 2007: part II: Unsettled issues. Circulation 116, 961–968 (2007).
- Rubboli A, Milandri M, Castelvetri C et al.: Meta-analysis of trials comparing oral anticoagulation and aspirin versus dual antiplatelet therapy after coronary stenting. Cardiology 104, 101–106 (2005).
- Peters RJ, Mehta SR, Fox KA et al.: Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Major Bleeding Angina to Prevent Recurrent Events (CURE) study. Circulation 108, 1682–1687 (2003).
- Chen ZM, Jiang LX, Chen YP et al.: Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 366, 1607–1621 (2005).
- Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E: The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126, 204S–233S (2004).
- The ACTIVE Writing Group on behalf of the ACTIVE Investigators: Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 367, 1903–1912 (2006).
- Nguyen M, Lim Y, Walton A et al.: Combining warfarin and antiplatelet therapy after coronary stenting in the Global Registry of Acute Coronary Events: is it safe and effective to use just one antiplatelet agent? Eur. Heart J. 28, 1717–1722 (2007).
- Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S: Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 115, 2689–2696 (2007).
- Donadini MP, Douketis JD: Combined warfarin-aspirin therapy: what is the evidence for benefit and harm and which patients should (and should not) receive it? J. Thromb. Thrombolysis 29(2), 208–213 (2010).
- Sørensen R, Hansen ML, Abildstrom SZ et al.: Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet 374, 1967–1974 (2009).
- Subherwal S, Bach RG, Chen AY et al.: Baseline risk of major bleeding in non-STsegment- elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of UnsMajor bleeding angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 119, 1873–1882 (2009).
- Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL: Combining antiplatelet and anticoagulant therapies. J. Am. Coll. Cardiol. 54, 95–109 (2009).
- Rubboli A, Colletta M, Herzfeld J, Sangiorgio P, Di Pasquale G: Periprocedural and medium-term antithrombotic strategies in patients with an indication for long-term anticoagulation undergoing coronary angiography and intervention. Coron. Artery Dis. 18, 193–199 (2007).
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ: Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 285, 2864–2870 (2001).
- Rao SV, Eikelboom J, Steg PG et al.: Standardized reporting of bleeding complications for clinical investigations in acute coronary syndromes: a proposal from the Academic Bleeding Consensus (ABC) multidisciplinary working group. Am. Heart J. 158, 881–886 (2009).
- Rossini R, Musumeci G, Lettieri C et al.: Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am. J. Cardiol. 102, 1618–1623 (2008).
- Graham I, Atar D, Borch-Johnsen K et al.: European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur. J. Cardiovasc. Prev. Rehabil. 14, S1–S113 (2007).
- Tay KH, Lane DA, Lip GY: Bleeding risks with combination of oral anticoagulation plus antiplatelet therapy: is clopidogrel any safer than aspirin when combined with warfarin? Thromb. Haemost. 100, 955–957 (2008).
- Wells P, Forgie M, Simms M et al.: The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch. Intern. Med. 163, 917–920 (2003).
- Beyth R, Quinn L, Landefeld C: Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am. J. Med. 105, 91–99 (1998).
- Fuster V, Ryden LE, Cannom DS et al.: ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Eur. Heart J. 27, 1979–2030 (2006).
- National Collaborating Centre for Chronic Conditions: Atrial fibrillation: national clinical guideline for management in primary and secondary care. Royal College of Physicians, London, UK (2006).
- Becker RC, Meade TW, Berger PB et al.: The primary and secondary prevention of coronary artery disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133, S776–S814 (2008).
- Lip GY, Huber K, Andreotti F et al.: Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/stenting. Thromb. Haemost. 103, 13–28 (2010).
- Popma JJ, Berger P, Ohman EM et al.: Antithrombotic therapy during percutaneous intervention. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 126, 576S–599S (2004).
- Rossini R, Musumeci G, Lettieri C et al.: Long-term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am. J. Cardiol. 102, 1618–1623 (2008).
- Bhatt DL, Scheiman J, Abraham NS et al.: ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 118, 1894–1909 (2008).
- Ho PM, Maddox TM, Wang L et al.: Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 301, 937–944 (2009).
- Khurram Z, Chou E, Minutello R et al.: Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J. Invasive Cardiol. 18, 162–164 (2006).
- Lip GY, Karpha M: Anticoagulant and antiplatelet therapy use in patients with atrial fibrillation undergoing percutaneous coronary intervention: the need for consensus and a management guideline. Chest 130, 1823–1827 (2006).
- DeEugenio D, Kolman L, DeCaro M et al.: Risk of major bleeding with concomitant dual antiplatelet therapy after percutaneous coronary intervention in patients receiving long-term warfarin therapy. Pharmacotherapy 27, 691–696 (2007).
- Manzano-Fernández S, Pastor F, Marín F et al.: Increased major bleeding complications related to triple antithrombotic therapy usage in patients with atrial fibrillation undergoing percutaneous coronary artery stenting. Chest 134, 559–567 (2008).
- Ruiz-Nodar J, Marín F, Hurtado J et al.: Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J. Am. Coll. Cardiol. 51, 818–825 (2008).
- Manzano-Fernandez S, Marin F, Pastor-Perez FJ et al.: Impact of chronic kidney disease on major bleeding complications and mortality in patients with indication for oral anticoagulation undergoing coronary stenting. Chest 135, 983–990 (2009).
- Halbfass P, Janko S, Dorwarth U, Riess G, Antoni G, Hoffmann E: Dilemma of antithrombotic therapy in anticoagulated atrial fibrillation patients squeezed between thrombosis and bleeding events: a single-center experience. Europace 11, 957–960 (2009).
- Halg C, Brunner-La Rocca HP, Kaiser C et al.: Early and late incresed bleeding rates after angioplasty and stenting due to combined antiplatelet and anticoagulanttherapy. EuroInterv. 5, 425–431 (2009).
- Gilard M, Blanchard D, Helft G et al.: Antiplatelet therapy in patients with anticoagulants undergoing percutaneous coronary stenting (from Stenting and Oral Anticoagulants [STENTICO]). Am. J. Cardiol. 104, 338–342 (2009).
- Management of patients with atrial fibrillation undergoing coronary artery stenting (AFCAS). http://clinicaltrials.gov/ct2/show/ NCT00596570
- LASER: Real Life Antithrombotic Stent Evaluation Registry. http://clinicaltrials.gov/ct2/show/ NCT00865163
- Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation (ISAR-TRIPLE). http://clinicaltrials.gov/ct2/show/ NCT00776633.
- What is the Optimal Antiplatelet and Anticoagulant in Patients with Oral Anticoagulation and Stenting (WOEST) study. www.clinicaltrials.gov/ct2/show/ NCT00769938
▪ Most comprehensive case–control analysis of outcomes in warfarin-treated patients.
▪ Recent recommendation on antithrombotic treatments after stenting.
▪ Largest database on the wide variation in antithrombotic strategies in this patient group.
▪ New comprehensive review on the net effects of warfarin–aspirin therapy.
▪ New nationwide data on the bleeding risks of antithrombotic treatments.
▪ Most recent recommendation on treatment strategies in patients with atrial fibrillation undergoing coronary stenting.
▪ Websites