Review Article - Neuropsychiatry (2017) Volume 7, Issue 5
Depression and Vitamin D Deficiency: Causality, Assessment, and Clinical Practice Implications
- Corresponding Author:
- Alessandro Cuomo, M.D
Division of Psychiatry, University of Siena, Viale Bracci 1, 53100 Siena, Italy
Tel: +39- 0577-586275
Fax: +39-0577-233451
Abstract
A literature review was conducted to investigate the relationship between low vitamin D concentration and depression and to report on the current knowledge about the assessment and treatment of low vitamin D in individuals with depression. Our literature review found substantial evidence for a significant relationship between depression and vitamin D deficiency but also highlighted the need for more studies to establish the direction of causality in the association between vitamin D deficiency and depression, and to determine the best preventative and treatment strategies for hypovitaminosis D in patients with depression.
Keywords
Vitamin D, Deficiency, Depression, Assessment, Treatment
Introduction
The possibility of a role of vitamin D in psychiatric disorders is suggested by regionspecific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, substantia nigra, amygdala, and hippocampus [1]. Many of these regions also express 1α-hydroxylase enzymes able to metabolize 25(OH)D to 1,25(OH)2D3, which suggests the possibility for vitamin D to play an autocrine or paracrine action in the brain [2]. Indeed, vitamin D may play a key role in the pathophysiology of depression and a number of studies have shown the presence of vitamin D, its receptors (VDR) and associated enzymes (CYP 24A1, CYP 27B1) in several regions of the brain, pointing to a role of vitamin D as a neuroactive/neurosteroid hormone involved in key functions such as neuroprotection, neuroimmunomodulation, brain development and regular brain function [2,3]. Furthermore, there is emerging evidence of possible neuroprotective roles that vitamin D may play through its effects on inflammation [4-6]. Certainly, growing body of data suggests that upregulation of proinflammatory cytokines in the brain may be associated with depression [5] and vitamin D may well be one of modulator in the association between depression and inflammatory response through its effect on the immune system [7,8].
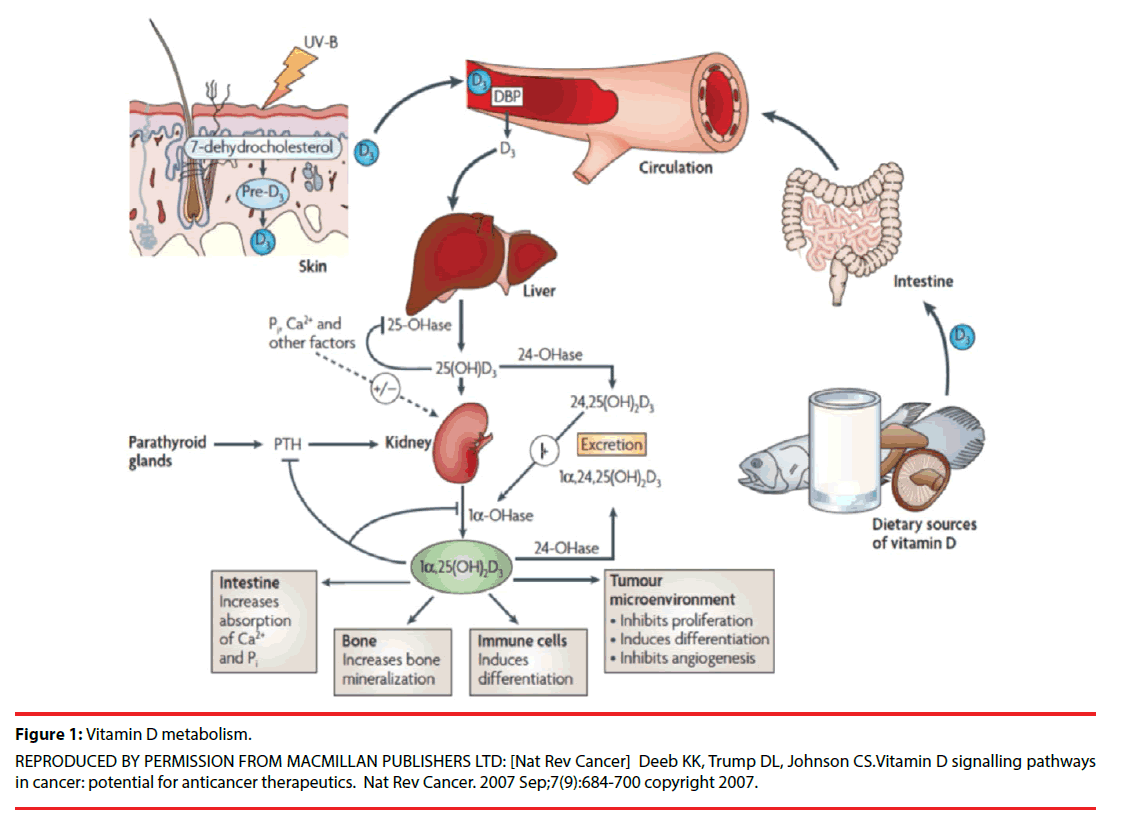
Vitamin D is built from the action of ultraviolet B sun rays (UVB) on dietary sources (Figure 1).
Figure 1: Vitamin D metabolism.
REPRODUCED BY PERMISSION FROM MACMILLAN PUBLISHERS LTD: [Nat Rev Cancer] Deeb KK, Trump DL, Johnson CS.Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007 Sep;7(9):684-700 copyright 2007.
Dietary intake of vitamin D is necessary to reach adequate levels of vitamin D and the best natural sources of vitamin D (include fatty fish such as salmon, tuna, and mackerel whereas smaller amount may be provided by cheese, beef liver, and egg yolks (Table 1). A newer and interesting strategy to boost vitamin D consists of exposing vegetables to ultraviolet light.
| Food | IUs per serving* | Percent DV** |
|---|---|---|
| Cheese, Swiss, 1 ounce | 6 | 2 |
| Ready to eat cereal, fortified with 10% of the DV for vitamin D, 0.75 1 cup (more heavily fortified cereals might provide more of the DV) | 40 | 10 |
| Egg, 1 large (vitamin D is found in yolk) | 41 | 10 |
| Liver, beef, cooked, 3 ounces | 42 | 11 |
| Sardines, canned in oil, drained, 2 sardines | 46 | 12 |
| Margarine, fortified, 1 tablespoon | 60 | 15 |
| Yogurt, fortified with 20% of the DV for vitamin D, 6 ounces (more heavily fortified yogurts provide more of the DV) | 80 | 20 |
| Milk, nonfat, reduced fat, and whole, vitamin D-fortified, 1 cup | 115-124 | 29-31 |
| Orange juice fortified with vitamin D, 1 cup (check product labels, as amount of added vitamin D varies) | 137 | 34 |
| Tuna fish, canned in water, drained, 3 ounces | 154 | 39 |
| Salmon (sockeye), cooked, 3 ounces | 447 | 112 |
| Swordfish, cooked, 3 ounces | 566 | 142 |
| Cod liver oil, 1 tablespoon | 1,360 | 340 |
Modified From: Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010. Accessed at https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ on March 18, 2017
Table 1: Selected Food Sources of Vitamin D.
In the United States, most of the vitamin D is provided for by fortified food, which includes orange juice, milk, cheese, cereals, yogurt, margarine, and soy beverages. However, the amount of vitamin D varies across different food and brands and different servings are to be consumed for different food and beverages in order to reach the minimum daily requirement of vitamin D [9,10]. Fortified food may either contain vitamin D2 or vitamin D3, which has shown greater efficacy in increasing blood 25-hydroxyvitamin D (25[OH]D) concentration compared to vitamin D2 [11].
The best strategy to evaluate vitamin D status is by a 25-hyroxyvitamin D (25-OH D) blood level, drawn after a 3-month period of a stable regimen of vitamin D intake [12-15]. Serum 25(OH)D is considered the standard measure for vitamin D status since it is the main circulating and most stable form of vitamin D. Risk factors for vitamin D deficiency include conditions that affect cutaneous production (such as inadequate sunlight exposure, using sunscreen or protective clothing or dark skin pigmentation), as well as aging, obesity, renal or gastrointestinal dysfunctions and the use of certain medications [10,16-18] (Table 2).
| Age (>65) |
| Insufficient sunlight |
| Dark skin |
| Breastfeeding |
| Renal disease |
| Hepatobiliary disease |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Obesity (BMI >30 kg/m2 |
| Malabsorption disease |
Table 2: Risk factors associated with vitamin D deficiency.
It has been estimated that over one billion people have either vitamin D insufficiency or deficiency. Vitamin D deficiency is defined as a condition with by a vitamin D level that is less than 20 nmol/L, whereas vitamin D insufficiency is defined as a vitamin D level of less than 30 nmol/L [10,19,20]. Excessive or even toxic vitamin D levels (hypervitaminosis D) may occur when 25(OH)D levels exceed 100 nmol/L (Table 3).
| nmol/L** | ng/mL* | Health status |
|---|---|---|
| >125 | >50 | Emerging evidence links potential adverse effects to such high levels, particularly >150 nmol/L (>60 ng/mL) |
| ≥50 | ≥20 | Generally considered adequate for bone and overall health in healthy individuals |
| 30 to < 50 | 12 to <20 | Generally considered inadequate for bone and overall health in healthy individuals |
| <30 | <12 | Associated with vitamin D deficiency, leading to rickets in infants and children and osteomalacia in adults |
** 1 nmol/L = 0.4 ng/mL
Modified From: Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.
Accessed at https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ on March 18, 2017
Table 3: Serum 25-Hydroxyvitamin D [25(OH)D] Concentrations and Health*.
Adequate vitamin D intake (AI) is considered to be between 200 and 600 IU/day for both genders, from the very first years of age to the age of 50. The required intake rises to 600 IU/day for people between 51-70 years; and to 800 IU/ day for people older than 70 years [9]. A recent recommendation from the American Academy of Pediatrics increased the suggested daily intake of vitamin D for infants, children, and adolescents to 400 IU/day [21]. Values of dietary reference intakes according to Institute of Medicine, Food and Nutrition Board are reported in Table 4.
| Age | Male Female | Pregnancy | Lactation |
|---|---|---|---|
| >70 years | 800 IU (20 mcg) 800 IU (20 mcg) | ||
| 51-70 years | 600 IU (15 mcg) 600 IU (15 mcg) | ||
| 19-50 years | 600 IU (15 mcg) 600 IU (15 mcg) | 600 IU (15 mcg) | 600 IU (15 mcg) |
| 14-18 years | 600 IU (15 mcg) 600 IU (15 mcg) | 600 IU (15 mcg) | 600 IU (15 mcg) |
| 1-13 years | 600 IU (15 mcg) 600 IU (15 mcg) | ||
| 0-12 months* | 400 IU (20 mcg) 400 IU (20 mcg) |
Table 4: Recommended Dietary Allowances (RDAs) for Vitamin D per day.
Although several studies have pointed to a relatively strong relationship between depression and vitamin D deficiency, several aspects remain unclear and controversial. To this end, we decided to conduct a narrative review on the relationship between low levels of vitamin D and depression and on the best preventative and treatment strategies for hypovitaminosis D in patients with depression.
Methods
We conducted a narrative review of all publications related to Vitamin D and psychiatric disorders [Keywords: “vitamin D”, “Deficiency”, “Depression”, “Assessment”, “Treatment” (and their synonyms)]. The following search databases were queried: PubMed; Medline, Cochrane Library (up to 2017). Additional publications worthy of interest were extracted from the bibliography of each study primarily consulted.
Results
▪ Vitamin D and Depression
The Third National Health and Nutrition Examination Survey [22], which enrolled a sample of 7,970 non-institutionalized U.S. residents age 15 to 39, confirmed that people with serum vitamin D ≤50 nmol/L are at a significantly higher risk of showing depression than individuals whose serum levels of vitamin D are greater or equal to 75 nmol/L. For instance, a study of 1,282 adults age 65 to 95 in the Netherlands [23] found that depressed subjects showed 25(OH)D levels that were 14% lower than controls. Also, a relationship was found between severity of depression and low 25 (OH)D serum levels, which remained significant after adjusting for age, gender, smoking status, body mass index, and number of comorbid chronic illnesses. Reduced 25-hydroxyvitamin D and elevated parathyroid hormone (PTH) serum levels have been associated with depressive symptoms in various clinical settings. Of interest, an inverse relationship between 25(OH)D serum levels and depression, has been shown even after having accounted and controlled for several lifestyle and health factors among communitydwelling European men [24].
The relationship between depression and vitamin D deficiency has also been investigated in the older population and/or in subjects with medical comorbidities [25,26]. Several studies showed a significant relationship between vitamin D deficiency and late-life depression in northern latitudes [27]. In further assessment of an older population-based cohort living at northern latitudes [28], a moderate inverse relationship between vitamin D serum level and depressive symptoms was observed among both genders. Furthermore, older men with low vitamin D levels (<30 nmol/l) were twice as likely to be depressed at the time of the assessment compared with similarly aged men whose vitamin D blood levels were adequate (≥50 nmol/l), even after controlling for factors such as hypertension and diabetes, which may contribute to depression as well. Interestingly, no significant relationship was found between vitamin D levels and current depression among women. Finally, high vitamin D serum levels were found to be protective against the development of poststroke Depression [PSD] [29]. Conversely, a relationship was found between low vitamin D serum level and the development of PSD, as well as an association was found between Vitamin D levels at hospital intake and PTS development at 1-month post-stroke.
A recent large cohort study showed an association between low vitamin D levels and both presence and severity of depression, this suggesting the possibility that hypovitaminosis D indicates an underlying biological susceptibility for depression [30].
A cross-sectional study [31] involving 80 elderly subjects (40 individuals with mild Alzheimer’s and 40 without Alzheimer’s or other types of dementio), all aged between 60 and 92 years, showed that 58% of the entire sample had abnormally low vitamin D levels. In addition, a relationship between vitamin D deficiency and the presence of a current mood disorder, as assessed by the depressive symptoms inventory, was found. In another study, 69% of 75 individuals with fibromyalgia were diagnosed with deficient or insufficient serum levels of vitamin D. Depression was higher (using the Hospital and Anxiety Depression Scale [HADS] [32] Median = 31) for those individuals with vitamin D deficiency than for those with insufficient (HADS = 22.5) or normal (HADS = 23.5) levels of vitamin D [33]. Similar results were found in a group pf subjects with secondary hyperparathyroidism (n = 21), in whom lower serum vitamin D levels were significantly related to higher scores on the Beck Depression Inventory [BDI] [34] as compared to a control group [35].
A negative correlation between Vitamin D3 serum levels and clinically significant depressive symptoms detected across five weekly assessments was found in a group of young adult healthy women [36]. These findings indicated that black young women were more likely to show vitamin D insufficiency and more likely to be depressed than other women, this being in line with the results of prior research [19,37,38]. Robinson, et al. [39] reported on the relationship between vitamin D levels during pregnancy and the development of post-partum depression [PPD]. Interestingly, low serum vitamin D levels during pregnancy resulted to be a risk factor for the development of postpartum depression symptoms.
Similar results were found by Murphy, et al. [40] who evaluated the relationship between vitamin D levels and depressive symptoms in a sample of 97 women, who were assessed on a monthly basis, for the first seven months of the postpartum period. In this study, women with lower vitamin D levels consistently showed higher rates of depression as compared to women with higher vitamin D levels. Two additional studies pointed to a negative and significant correlation between vitamin D serum levels in the first trimester of gestation and the presence of depressive symptoms in the second trimester [41,42]. Moreover, the relationship between vitamin D levels in the second pregnancy trimester and postpartum depression during the first six months after pregnancy was investigated [43]. This study showed that lower maternal levels of 25-hydroxy-vitamin D3 in the second trimester of pregnancy were correlated with higher degree depressive symptoms at one week, six weeks, and six months of the postpartum period.
Seasonal changes in the levels of vitamin D suggest the possibility that supplementation may help patients who have seasonal mood disorders. In a randomized, double-blind study [44], 44 healthy individuals received vitamin D3, at a dose of 400 IU/d, or vitamin D3 at the dose of 800 IU/d, or no vitamin D3 for five days during late winter. Based on selfreports, vitamin D3 significantly enhanced positive affect, and there was some evidence it reduced negative affect. In a pilot study [45] of 9 women with vitamin D serum levels lower than 40 ng/ml, a supplementation during winter with vitamin D was associated with an average 10-point improvement in the scores of Beck Depression Inventory-II [46]. In a prospective RCT of 15 individuals with the seasonal affective disorder, all patients who received vitamin D supplementation improved in all outcome measures [47]. Vieth [48] randomized 82 adults with vitamin D deficiency to 600 IU/d or 4,000 IU/d of vitamin D3 for a period of three months, over two consecutive winters. Patients that received the higher dose showed some evidence of improved well-being compared to those that were assigned to the lower dose, although results did not reach statistical significance for all comparisons. Consistently with previous research, Berk, et al. [49] found that vitamin D deficiency may be associated to depression and other mental disorders and suggested a possible role of vitamin D as a treatment of depression, in augmentation to antidepressant agents. Another recent report [50] examined several studies on vitamin D and mood disorders in women and concluded that vitamin D may be an important contributor to mental and physical well-being in women.
A study in children suggested that the association between 25(OH)D3 serum levels and depression may well apply to children too [51]. In a case series [52] involving 48 adolescents with depression and vitamin D-deficiency subjects were given vitamin D3 over 3 months and a significant improvement in depressive symptoms, irritability, fatigue and well-being was observed.
Although most studies confirm the hypothesis that low vitamin D concentration is associated with depression, there has been research that has failed to show such a relationship. For instance, a large epidemiologic study in China [53] did not identify a relationship between vitamin D and depression in 3,262 men and women age 50 to 70.
Also, there is not a complete agreement concerning the potential use of vitamin D as a supplement for depression. For instance, two trials did not report a significant improvement in seasonal affective disorder (SAD) symptoms after vitamin D treatment [54,55] . Similarly a systematic review [56] concluded that there is insufficient data to support the effectiveness of Vitamin D to treat depressive symptoms in patients without vitamin D deficiency. In another review [57], low vitamin D levels were correlated with depressive symptoms but the impact on depression of adding vitamin D supplements was deemed as uncertain.
Kjærgaard, et al. [58] systematically analyzed vitamin D levels in a case-control study supported by a vitamin D supplementation randomized controlled trial (RCT). In the casecontrol phase, subjects with low baseline 25(OH) D concentrations resulted to be significantly more depressed than subjects with high 25(OH) D levels. Individuals with low 25(OH)D levels were randomized to receiving either vitamin D3 supplementation of 40,000 IU per week or placebo, for a total of 6 months. Although low levels of vitamin D resulted significantly correlated with depressive symptoms, vitamin D supplementation failed to show a meaningful effect on depressive symptoms. This implies the possibility that vitamin D deficiency may be the effect of depression rather than being the cause of depression. In a double-blind, controlled trial, Jorde, et al. [59] randomized 441 individuals age 21 to 70 to vitamin D 20,000 IU, vitamin D 40,000 IU per week; or placebo for 1 year. The study results showed that individuals with 25(OH)D serum levels lower than 40 nmol/L scored significantly higher on depression rating scales than those with 25(OH)D serum levels ≥40 nmol/L. No significant change in depression ratings was observed in the placebo group. These results must be interpreted with caution because depressive symptoms were secondary endpoints in this study.
Overall, the results of our literature search highlights the need for more prospective randomized controlled trials of vitamin D for the prevention and/or treatment of depression, as well as the need to better define the relationship of causality between depression and vitamin D deficiency.
▪ Assessment, Treatment and Practice Implications
The total 25-hydroxyvitamin D (25-OH-VitD) level (i.e., the sum of the amount of 25-OHvitamin D2 and 25-OH-vitamin D3) is the appropriate indicator of vitamin D body stores. The National Institutes of health (NIH) [60] Office of Dietary Supplements (ODS) recently (November 2010) established a worldwide collaborative effort to standardize the laboratory measurement of vitamin D status, which was called Vitamin D Standardization Program (VDSP). The VDSP goal is to promote the detection, evaluation, and treatment of vitamin D deficiency and insufficiency through an improvement in the standardized laboratory measurement of total 25-hydroxyvitamin D [25(OH)D] serum levels. Specific recommendation to make it accurate and equivalent over time, location, and laboratory procedure were provided.
Albeit there is no universal consensus about a treatment cut-point, investigations suggest 25 to 35 nmol/L as the minimum concentration of 25-OH-VitD required to avoid the adverse effects of deficiency [61-63]. Findings of our review indicate that low 25(OH)D serum levels may be associated to mood disorders, pointing out the possibility of common biologic mechanisms underpinning the two conditions. Once the association of vitamin D deficiency and mood disorders is clearly confirmed and the direction of causality has been studied, the possibility of primary prevention should be considered. However, as of today there is not enough research evidence to recommend a change in clinical practice recommendations for vitamin D supplementation as a strategy to prevent mood disorders. Indeed, more studies are needed to determine whether vitamin D deficiency is one of the risk factors for mood disorders or it is more an effect than a cause of these diseases. Nevertheless, it is well known that vitamin D deficiency is associated to several physical illnesses and hence it seems reasonable to recommend that 25(OH) D serum levels be kept in the normal range [19,20].
Depressive symptoms and reduced physical functioning are prevalent and cause significant individual and societal burden [64]. Efficient, low-cost prevention strategies such as a monitoring and treatment of hypovitaminosis D would be necessary. However, no sufficient data has been accumulated yet to recommend vitamin D supplementation as a specific tool to prevent mood disorders. Of interest, Holick [65] reported that sun exposure (arms and legs for five to ten minutes; two or three times/week) might be beneficial for keep vitamin D levels within normal limits. Encouraging people to exercise outdoors during daylight hours would bring the benefit of ensuring an adequate level of vitamin D while possibly contributing to mood improvement. However, the benefits of outdoor exercise during the hours that allow exposure to natural sunlight need to be weighed against the risk of skin cancer, which once again highlights how difficult it is to make universal recommendations. For some people, sunshine exposure or diet alone may not be enough to provide adequate amounts of vitamin D. Therefore, any intervention to treat vitamin D deficiency and vitamin D supplementation should be provided under appropriate medical supervision and monitoring. Daytime outdoor physical activity may be considered as a surrogate indicator for sun exposure. Of interest it has been observed that exercise in itself may contribute to the maintenance of vitamin D levels, with mechanisms other than merely by increasing exposure of skin to sunlight [66,67]. Indeed, together with calcium supplement intake, the major modifiable predictors of vitamin D deficiency were body mass index (BMI) greater than 30 kg/m2 and low physical activity [68], which are both conditions that are frequently observed in patients with depression. Of note, it has been clearly shown that physical activity improves bone mass, reduces calcium elimination and raises the efficiency of its absorption [69], thus resulting in increased calcium serum levels, which translates into vitamin D sparing. Moreover, physical exercise and activity may increase the rate of lipolysis, decreased body weight and body fat stores and hence induce vitamin D mobilization from adipose tissue, thus increasing its blood levels [70-73].
Discussion
Although the amount of research about the relationship between vitamin D deficiency and depression is growing, several aspects remain unclear. For instance, it is not always clear whether vitamin D deficiency may be the result or the cause of depression. Subjects with depression may be more likely to develop low vitamin D because of lower outdoor activity or reduced nutrients intake. Conversely, the relationship of causality could well be in the opposite way. Indeed, the identification of vitamin D receptors in areas of the brain that have been associated to the development of depression strengthens the plausibility of a relationship between vitamin D and depression. Also, it remains to be established if adding vitamin D supplements may prevent and/or treat depression in individuals with vitamin D deficiency. Indeed, studies on vitamin D and depression and on the role of vitamin D supplementation have produced mixed results. This may be due to several reasons, including the use of different doses of vitamin D supplements for different lengths of time across different studies, the use of different parameters to define vitamin D deficiency, the use of different psychometric instruments to measure depression and mental health, and the administration of vitamin D at different frequencies (i.e., every day, once a week or once a month). Due to the methodological differences across different studies, it is difficult to establish the exact role of vitamin D in preventing or treating depression.
Conclusions
Study research shows a relationship between vitamin D deficiency and symptoms of depression. However, it remains unclear if low vitamin D levels are the cause or the effect of depression. Several factors contribute to hamper the studies on the relationship between Vitamin D and depression, including the fact that lack of vitamin D is just one of the many factors that may contribute to depression. Also, the effects of vitamin D on depression may take a long time to work, this asking that any study to evaluate this relationship covers an adequately long period of time. Also, further research is needed to define appropriate protocols for vitamin D testing and supplementation in clinical practice and to establish if, when and how much vitamin D supplementation can improve depression. Clearly, eating food that is rich of vitamin D, taking dietary supplements to improve vitamin D deficiency, and spending time in the sunshine and/ or exercising outdoors may improve mental well-being in patients with depression. Although several issues in the relationship between depression and low levels of vitamin D remain controversial and are in need of further studies, the literature is already providing enough data to recommend screening for and treating vitamin D deficiency in subjects with depression, which is easy, cost-effective and may improve depression outcome.
Financial support
None.
References
- Prufer K, Veenstra TD, Jirikowski GF, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J. Chem. Neuroanatomy 16(2), 135-145 (1999).
- Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanatomy 29(1), 21-30 (2005).
- Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr. Opin. Clin Nutrit. Metab.Care 10(1), 12-19 (2007).
- Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing "D"ecline? Mol. Aspects. Med29(6), 415-422 (2008).
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35(3), 760-768 (2011).
- Zittermann A, Dembinski J, Stehle P. Low vitamin D status is associated with low cord blood levels of the immunosuppressive cytokine interleukin-10. Pediatr. Allergy. Immunol 15(3), 242-246 (2004).
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol 8(9), 685-698 (2008).
- van Etten E, Stoffels K, Gysemans C, et al. Regulation of vitamin D homeostasis: implications for the immune system. Nutr. Rev 66(10 Suppl 2), S125-34 (2008).
- Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) National Academy of Sciences (2011).
- Holick MF. Vitamin D deficiency. N. Engl. J. Med 357(3), 266-281 (2007).
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab 89(11), 5387-5391 (2004).
- Holick MF. Chapter 20: Vitamin D. In: Shils ME SM, Ross AC, Caballero B, Cousins RJ, editors. Modern nutrition in health and disease. 10th ed. Baltimore, MD: Lippincott Williams and Wilkins; 376–395 (2006).
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr 69(5), 842-856 (1999).
- Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr 73(2), 288-294 (2001).
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr 77(1), 204-210 (2003).
- Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta. dermato-venereologica 91(2), 115-124 (2011).
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr 87(4), 1080s-6s (2008).
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr 79(3), 362-371 (2004).
- Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr 135(2), 317-322 (2005).
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am. J. Clin. Nutr 85(3), 649-650 (2007).
- Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122(5), 1142-1152 (2008).
- Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int. Arch. Med 3(1), 29 (2010).
- Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch. Gen. Psychiatry 65(5), 508-512 (2008).
- Lee DM, Tajar A, O'Neill TW, et al. Lower vitamin D levels are associated with depression among community-dwelling European men. J. psychopharmacol 25(10), 1320-1328 (2011).
- May HT, Bair TL, Lappe DL, et al. Association of vitamin D levels with incident depression among a general cardiovascular population. Am. Heart. J 159(6), 1037-1043 (2010).
- Lapid MI, Cha SS, Takahashi PY. Vitamin D and depression in geriatric primary care patients. Clin. Interv. Aging 8(1), 509-514 (2013).
- Stewart R, Hirani V. Relationship between vitamin D levels and depressive symptoms in older residents from a national survey population. Psychosom. Med 72(7), 608-612 (2010).
- Imai CM, Halldorsson TI, Eiriksdottir G, et al. Depression and serum 25-hydroxyvitamin D in older adults living at northern latitudes - AGES-Reykjavik Study. J. Nutr. Sci 4(1), e37 (2015).
- Han B, Lyu Y, Sun H, et al. Low serum levels of vitamin D are associated with post-stroke depression. Eur. J. Neurol 22(9), 1269-1274 (2015).
- Milaneschi Y, Hoogendijk W, Lips P, et al. The association between low vitamin D and depressive disorders. Mol. Psychiatry 19(4), 444-451 (2014).
- Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am. J. Geriatr. Psychiatry 14(12), 1032-1040 (2006).
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta. Psychiatr. Scand 67(6), 361-370 (1983).
- Armstrong DJ, Meenagh GK, Bickle I, et al. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin. Rheumatol 26(4), 551-554 (2007).
- Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch. Gen. Psychiatry 4(1), 561-571 (1961).
- Jorde R, Waterloo K, Saleh F, et al. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. The Tromso study. J. Neurol 253(4), 464-470 (2006).
- Kerr DC, Zava DT, Piper WT, et al. Associations between vitamin D levels and depressive symptoms in healthy young adult women. Psychiatry. Res 227(1), 46-51 (2015).
- Eisenberg D, Hunt J, Speer N. Mental health in American colleges and universities: variation across student subgroups and across campuses. J. Nerv. Ment. Dis 201(1), 60-67 (2013).
- Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am. J. Clin. Nutr 67(6), 1108-1110 (1998).
- Robinson M, Whitehouse AJ, Newnham JP, et al. Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch. Womens. Ment. Health 17(3), 213-219 (2014).
- Murphy PK, Mueller M, Hulsey TC, et al. An exploratory study of postpartum depression and vitamin d. J. Am. Psychiatr. Nurses. Assoc 16(3), 170-177 (2010).
- Cassidy-Bushrow AE, Peters RM, Johnson DA, et al. Vitamin D nutritional status and antenatal depressive symptoms in African American women. J. Womens. Health. (Larchmt) 21(11), 1189-1195 (2012).
- Brandenbarg J, Vrijkotte TG, Goedhart G, et al. Maternal early-pregnancy vitamin D status is associated with maternal depressive symptoms in the Amsterdam Born Children and Their Development cohort. Psychosom. Med 74(7), 751-757 (2012).
- Gur EB, Gokduman A, Turan GA, et al. Mid-pregnancy vitamin D levels and postpartum depression. Eur. J. Obstet. Gynecol. Reprod. Biol 179(1), 110-116 (2014).
- Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology 135(4), 319-323 (1998).
- Shipowick CD, Moore CB, Corbett C, et al. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl. Nurs. Res 22(3), 221-225 (2009).
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation (1996).
- Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J. Nutr. Health. Aging 3(1): 5-7 (1999).
- Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr. J3(1), 8 (2004).
- Berk M, Sanders KM, Pasco JA, et al. Med. Hypotheses 69(6), 1316-139 (2007).
- Murphy PK, Wagner CL. Vitamin D and mood disorders among women: an integrative review. J. Midwifery. Womens. Health 53(5), 440-446 (2008).
- Tolppanen AM SA, Fraser WD, Lewis G, et al. The association of serum 25-hydroxy vitamin D3 and D2 with depressive symptoms in childhood-a prospective cohort study. J. Child. Psychol. Psychiatry 53(7), 757-766 (2012)
- Hogberg G, Gustafsson SA, Hallstrom T, et al. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta. Paediatrica 101(7), 779-783 (2012).
- Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J. Affect. Disord 118(1-3), 240-243 (2009).
- Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry. Res 49(1), 77-87 (1993).
- Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J. Nutr. Health. Aging 10(2), 151-153 (2006).
- Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review protocol. Syst. Rev 2(1), 64 (2013).
- Shaffer JA, Edmondson D, Wasson LT, et al. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosomatic. Med 76(3), 190-196 (2014).
- Kjaergaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br. J. Psychiatry 201(5), 360-368 (2012).
- Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J. Intern. Med 264(6): 599-609 (2008).
- https://ods.od.nih.gov/Research/vdsp.aspx
- Heaney RP. Vitamin D: how much do we need, and how much is too much? Osteoporos. Int 11(7), 553-555 (2000).
- Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int 7(5), 439-443 (1997).
- Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J. Clin. Endocrinol. Metab 89(7), 3152-3157 (2004).
- World Health Organization. The Global Burden of Disease: 2004 Update. Geneva SWP (2008).
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr 80(6 Suppl), 1678s-88s (2004).
- Scragg R, Holdaway I, Singh V, et al. Serum 25-hydroxyvitamin D3 is related to physical activity and ethnicity but not obesity in a multicultural workforce. Aust. N Z J Med 25(3), 218-223 (1995).
- Wanner M, Richard A, Martin B, et al. Associations between objective and self-reported physical activity and vitamin D serum levels in the US population. Cancer. Causes. Control 26(6), 881-891 (2015).
- Brock K, Cant R, Clemson L, et al. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J. Steroid. Biochem. Mol. Biol 103(3-5), 786-792 (2007).
- Specker BL. Evidence for an interaction between calcium intake and physical activity on changes in bone mineral density. J. Bone. Miner. Res 11(10), 1539-1544 (1996).
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. Respir. Environ. Exerc. Physiol 56(4), 831-838 (1984).
- Riedt CS, Cifuentes M, Stahl T, et al. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J. Bone. Miner. Res 20(3), 455-463.
- Tzotzas T, Papadopoulou FG, Tziomalos K, et al. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J. Clin. Endocrinol. Metab 95(9), 4251-4257 (2010).
- Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr 89(5), 1321-1327 (2009).