Review Article - Interventional Cardiology (2014) Volume 6, Issue 1
Current trends in the treatment of venous thoracic outlet syndrome: a comprehensive review
- Corresponding Author:
- Anthony J Comerota
Jobst Vascular Institute, 2019 Hughes Drive
Suite 400, Toledo, OH 43606, USA
Tel: +1 419 291 2080
Fax: +1 419 479 6980
E-mail: anthony.comerotaMD@promedica.org
Abstract
The term ‘thoracic outlet syndrome’ (TOS) represents a variety of disorders caused by the compression of nerves, arteries and veins as they exit the thorax and enter the axilla. Three basic syndromes are reported according to the involved anatomic structures: neurogenic, venous and arterial. Occasionally, the neurological and arterial syndromes may coexist in the same patient. However, for ease of understanding, each of these syndromes should be conceptualized as distinct clinical entities for they have characteristic differences in clinical presentation, diagnostic workup and treatment. Venous TOS, which is the topic of this review, can be further divided into two categories, the thrombotic and the less common nonthrombotic venous TOS.
Keywords
axillosubclavian venous thrombosis, effort thrombosis, nonthrombotic venous thoracic outlet syndrome, Paget–Schroetter syndrome, thoracic outlet syndrome
The term ‘thoracic outlet syndrome’ (TOS) represents a variety of disorders caused by the compression of nerves, arteries and veins as they exit the thorax and enter the axilla. Three basic syndromes are reported according to the involved anatomic structures: neurogenic, venous and arterial. Occasionally, the neurological and arterial syndromes may coexist in the same patient. However, for ease of understanding, each of these syndromes should be conceptualized as distinct clinical entities for they have characteristic differences in clinical presentation, diagnostic workup and treatment. Venous TOS, which is the topic of this review, can be further divided into two categories, the thrombotic and the less common nonthrombotic venous TOS.
Thrombotic venous TOS, also known as Paget–Schroetter syndrome, specifically refers to primary axillosubclavian vein thrombosis [1]. Another term for this syndrome is ‘effort thrombosis’, as it is frequently associated with repetitive activities of arm elevation or exertion over a long period of time [2]. Sir James Paget first described a spontaneous thrombosis of the subclavian vein in 1875 [3], and in 1884 von Schroetter correlated this entity with direct damage of the vein caused by muscular strain [4]. The eponym ‘Paget–Schroetter syndrome’ first appeared in 1948 when it was coined by Hughes, an English surgeon who described this clinical condition in more detail [5].
Nonthrombotic venous TOS represents an intermittent/positional venous obstruction usually due to external compression, without the presence of intraluminal thrombus. Patients generally have normal venograms at rest, but develop varying degrees of extrinsic compression and venous collateral drainage with the arm abducted [6]. The natural history of these patients is not clear; however, some believe it may be a precursor of Paget–Schroetter syndrome.
This review focuses on the anatomy and pathophysiology associated with venous TOS, the diagnostic workup and proposed treatment. The venous variant of TOS in many ways is less controversial than the neurogenic variety and can be objectively identified by clinical examination, ultrasonography or venography. However, controversies remain regarding the most appropriate treatment, the need for thoracic outlet decompression, the role of thrombolytic therapy, and the indications for balloon angioplasty or stenting. Most importantly, significant controversy exists regarding the timing of treatment during the natural course of the disease. We describe treatment options and suggest an algorithm for appropriate, safe and effective patient management.
Epidemiology, anatomy & pathophysiology
Venous TOS accounts for approximately 5% of all TOS syndromes [7]. Axillosubclavian venous thrombosis is seen more often than nonthrombotic obstruction. Interestingly, only three reports have been published addressing the nonthrombotic clinical entity since 1973 [6,8,9]. In a 15-year review, 66 out of 87 patients operated on for TOS had subclavian vein thrombosis and 21 had obstruction without thrombosis [6]. Effort thrombosis is more widely reported. Its incidence in Sweden has been estimated at 2.03 per 100,000 people per year [10] and accounts for approximately 1–4% of all episodes of venous thrombosis [11,12]. The male-to-female ratio is approximately 2:1 and the mean age of presentation is the early 30s [10]. The right upper extremity is more commonly involved owing to the higher incidence of right-hand dominance and 60–80% of patients have a history of vigorous exercise of the involved extremity, such as heavy lifting, repetitive overhead motion or strenuous athletic activity [13].
The thoracic outlet is defined anatomically as two distinct spaces, the interscalene triangle (demarcated by the anterior and middle scalene muscles and the first rib) and the costoclavicular space (between the first thoracic rib and the clavicle) [14]. The interscalene triangle contains the brachial plexus and the subclavian artery. The costoclavicular space contains the subclavian vein alone. The subclavian vein crosses anterior to the anterior scalene muscle, passing adjacent to the junction of the clavicle and the first rib [15]. A third muscle, the subclavius, can also provide ‘bulk’ and narrow the costoclavicular space.
Theoretically, the subclavian vein can be compressed in this narrow passageway between bony structures (first rib and clavicle), muscular structures (hypertrophied anterior or middle scalene and subclavius muscle) or a bony and a muscular structure. Reported abnormalities include venous compression between the clavicle and first rib, between a hypertrophied scalene or subclavian tendon and first rib, between a scalene and a subclavian tendon, or by a congenital web [15]. However, it is not quite clear if the external compression of the subclavian vein originates from a narrow costoclavicular space, an abnormal bony structure, a hypertrophied muscle or from a combination of all of these since it has been demonstrated that the vein can be easily compressed even in normal individuals [16]. Nevertheless, whichever abnormality is responsible for the subclavian vein compression, the compression occurs or worsens when the arm is abducted [17,18]. Interestingly, this syndrome is often present in patients where the arm is overhead (painting, weight training and autorepair) or in athletes, such as baseball pitchers and swimmers [1]. More specifically, all of these activities are characterized by repetitive or prolonged vigorous hyperabduction or external rotation of the shoulder joint [19].
The exact mechanism of venous injury associated with the syndrome is not very well known. In the case of thrombosis, it is also unclear if thrombus is the result of a single insult or the cumulative effects of chronic injury to the vein. It has been hypothesized that repetitive and prolonged focal venous injury results in repeated trauma to both the intima and the wall of the vein itself. The intimal trauma results in intimal hypertrophy [20] and activation of the coagulation cascade [21], predisposing to thrombosis. The perivascular tissue develops inflammation, which in turn leads to fibrosis and scar tissue formation by dense collagen with persistent vein compression [22,23]. Lack of vein mobility due to the surrounding scar tissue increases the risk of vein trauma, producing a cycle of progressive injury whenever the diameter of the costoclavicular space changes. Perivenous fibrosis can be identified during the operative procedure to decompress the subclavian vein [1].
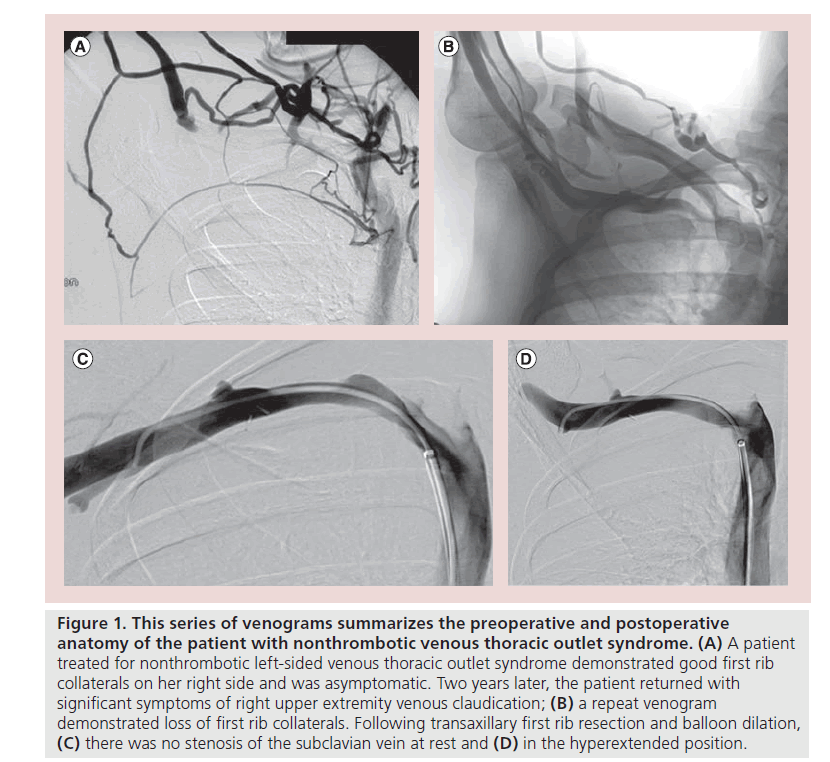
It seems that there is an initial phase of the syndrome that is characterized by intermittent venous outflow obstruction prior to vein injury. Patients may be asymptomatic due to the development of first rib collateral veins that facilitate drainage of the upper extremity. This condition has been described as nonthrombotic venous TOS and can be confirmed by a venogram showing the obstructed subclavian vein and its collateral veins when the arm is abducted [1]. On occasion, nonthrombotic TOS can progress to severe symptoms requiring decompression. This generally occurs as a result of the loss of collateral venous drainage, illustrated by Figure 1. Between the nonthrombotic state and the acute thrombotic condition (Paget–Schroetter syndrome) is another condition characterized by recurrent partial thrombosis followed by recanalization [16]. The alternation between thrombosis and recanalization causes local inflammation and scar formation leading to the development of venous webs and fibroelastic structures in the venous lumen. Eventually thrombus occludes the narrow segment of the subclavian vein due to stagnant flow. Propagation of the clot into the axillary vein compromises subclavian venous collaterals, resulting in the acute symptoms of effort thrombosis. When the thrombus is acute, it can be lysed, restoring patency to the central veins and first rib collaterals. If, however, a treatment strategy of thrombus removal is not used, chronic post-thrombotic venous obstruction can occur, resulting in an intraluminal fibrotic obstruction composed predominantly of collagen.
Figure 1: This series of venograms summarizes the preoperative and postoperative anatomy of the patient with nonthrombotic venous thoracic outlet syndrome. (A) A patient treated for nonthrombotic left-sided venous thoracic outlet syndrome demonstrated good first rib collaterals on her right side and was asymptomatic. Two years later, the patient returned with significant symptoms of right upper extremity venous claudication; (B) a repeat venogram demonstrated loss of first rib collaterals. Following transaxillary first rib resection and balloon dilation, (C) there was no stenosis of the subclavian vein at rest and (D) in the hyperextended position.
Clinical presentation & diagnosis
Arm pain, swelling and cyanosis are the typical clinical symptoms in patients with thrombotic or nonthrombotic venous TOS [24]. The non-thrombotic variety tends to resolve with rest. The constellation of signs and symptoms represent the upper extremity equivalent of phlegmasia cerulea dolens, which is the most common clinical entity in the case of acute iliofemoral vein occlusion of the lower extremity [25]. Any young, healthy and active individual presenting with these symptoms in the absence of indwelling central venous catheters should be suspected for venous TOS [26]. Arm edema is a unique symptom of venous obstruction, occurring in neither neurogenic nor arterial TOS. Pain, heaviness and achiness are often part of venous TOS, but are not uniformly present. Hand paresthesias can also be present and are usually the result of edema rather than nerve compression.
The nonthrombotic syndrome usually presents more insidiously than the thrombotic. The symptoms are intermittent, position-dependent [6,11] and usually elicited by either exercise or arm elevation. Their onset is gradual. In a series of 21 patients with nonthrombotic venous TOS, all patients but one had unilateral arm swelling [6]. The patient without swelling had severe cyanosis and subclavian vein occlusion when the arm was abducted to 90 degrees. Half of the patients had cyanosis and pain, and 16 out of 21 had occipital headache, neck pain or hand paresthesias.
Conversely, effort thrombosis is characterized by the sudden onset of symptoms usually within 24 h after the inciting event [7]. The symptoms are more pronounced than those of the nonthrombotic syndrome. The magnitude of arm swelling is quite significant; the affected arm can be as much as twice the size of the contralateral normal extremity [26]. Up to 80% of patients with effort thrombosis report a history of vigorous and prolonged limb exertion. Prominent superficial collateral veins may develop over the upper arm, anterior chest, and base of the neck, particularly when the occlusion is chronic [11,27].
The initial imaging test to confirm the clinical suspicion is usually ultrasonography [28]. It is inexpensive, readily available and noninvasive. The disadvantage is that it is technologist- dependent. Its sensitivity is reported to be 70–100% and its specificity 93% [29,30]. Its inability to visualize the central portion of the subclavian vein or to differentiate a central vein from a large collateral vein raises the falsenegative rate to 30% [1], making it an inadequate technique for excluding the diagnosis of subclavian vein thrombosis. Axial imaging techniques, such as computed tomography and magnetic resonance, are useful for examining the central veins [28]. They provide more anatomical information than venous duplex imaging and can accurately exclude the diagnosis of venous TOS.
Venography was previously the ‘gold standard’ for the diagnosis of venous TOS. The study can be performed via a peripheral vein, which may be difficult with an edematous limb. Occasionally the diagnosis can be missed if the upper arm cephalic vein is cannulated for contrast injection [31]. Sanders and Hammond reported a preference for the basilic rather than the cephalic vein for performing diagnostic venograms in patients with suspected nonthrombotic venous TOS syndrome [6]. Importantly, ultrasound-guided access to the deep vein system, either at the antecubital fossa or distal upper arm, provides the diagnosis while also providing catheter access for thrombolytic therapy, the initial treatment plan recommended for most patients with symptomatic effort thrombosis. However, deep veins are not typically accessed and the basilic vein is the most preferred access vein. Interestingly, some centers follow quite an aggressive diagnostic approach, proceeding to catheter-based venography as the most efficient and cost-effective approach for both diagnosis and treatment of the patient without relying on ultrasonographic or other noninvasive studies [26].
In the case of effort thrombosis, clinical diagnosis is generally evident. Ultrasound studies are now highly reliable for the diagnosis of acute axillosubclavian vein thrombosis. Imaging studies show occlusion of the subclavian vein at the costoclavicular junction, usually with thrombus extending into the axillary vein, with a rich pattern of venous collaterals around the occlusion. A patent vein with the presence of collaterals without intraluminal obstruction indicates nonthrombotic venous obstruction. If noninvasive studies demonstrate a patent central vein without collaterals, a venogram should be performed with the arm abducted to 90–180 degrees before ruling out venous TOS. In a series of 21 symptomatic patients with nonthrombotic venous TOS, Sanders and Hammond reported that abduction of the arm to 180 degrees and arm extension forward in a throwing position was necessary to demonstrate venous compression [6]. Venous compression during the above arm maneuvers can be observed in asymptomatic individuals; therefore, these occlusive findings with venography have a confirmative role in diagnosing venous TOS only in symptomatic patients [28].
Treatment
The standard of care among patients with acute deep venous occlusion of the upper extremity is anticoagulation alone. Thrombolysis, which is the alternative initial treatment, may theoretically reduce the rate of thromboembolic recurrences. However, there are no data from randomized controlled studies to support this hypothesis. Guzzo et al. imply that thrombolysis may not be a necessary step in the management of patients with effort thrombosis, since patency rates in patients who had anticoagulation alone, before surgical decompression, were similar with patients who had thrombolysis and subsequent decompression [32]. Moreover, the proponents of anticoagulation alone claim that severe post-thrombotic syndrome is almost never observed after conservative treatment of upper extremity deep venous thrombosis and the benefit from thrombolysis in the long term remains unclear [32].
Others have suggested a different approach based upon unfavorable patient outcomes. Most contemporary series are in favor of thrombolysis, suggesting that thrombotic occlusion of the axillosubclavian veins can lead to significant morbidity if treated with anticoagulation alone [33]. Considering that the mean age of the disease is the early thirties and the dominant arm is the one most likely to be affected, the impact on patients’ long-term quality of life can be significant. Treatment with arm elevation and anticoagulation alone is often inadequate for most patients with venous TOS. It is associated with chronic central venous obstruction resulting in significant post-thrombotic morbidity. Adams and DeWeese demonstrated that effort thrombosis resulted in residual venous obstruction in 78% of the cases [33]. Moreover, persistent symptoms and permanent disability were found in 91 and 68% of patients, respectively [33–35]. Acute pulmonary embolism can occur in 6–15% of patients [29,36]. Treatment algorithms that include early contrast venography with catheter-based thrombolytic therapy followed by surgical thoracic outlet decompression have demonstrated significantly improved outcomes [30,37,38].
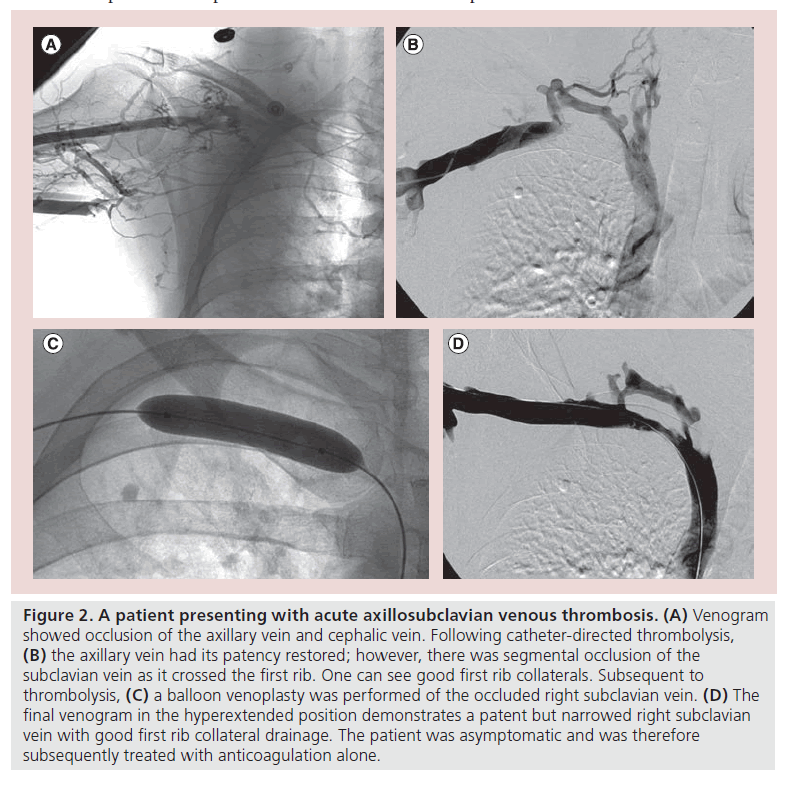
The current trend for treatment of extensive, acute axillosubclavian thrombosis in young, active individuals is a treatment strategy using catheter-based thrombolysis to clear the vein lumen followed by evaluation of the patient for first rib resection to correct the chronic subclavian vein compression (Figure 2). Therefore, for successful treatment of patients with thrombotic venous TOS, we need to consider three basic issues: early elimination of fresh thrombus, possible elimination of the cause of the subclavian vein compression and correction of residual subclavian vein stenosis.
Figure 2: A patient presenting with acute axillosubclavian venous thrombosis. (A) Venogram showed occlusion of the axillary vein and cephalic vein. Following catheter-directed thrombolysis, (B) the axillary vein had its patency restored; however, there was segmental occlusion of the subclavian vein as it crossed the first rib. One can see good first rib collaterals. Subsequent to thrombolysis, (C) a balloon venoplasty was performed of the occluded right subclavian vein. (D) The final venogram in the hyperextended position demonstrates a patent but narrowed right subclavian vein with good first rib collateral drainage. The patient was asymptomatic and was therefore subsequently treated with anticoagulation alone.
Thrombolytic therapy
In several centers, catheter-directed thrombolysis (CDT) has become the standard treatment to eliminate the acute thrombus in the axillosub-clavian veins [39,40]. Machleder emphasized the significance of successful thrombolysis, noting that 93% of patients with patent subclavian and axillary veins following successful thrombolysis and first rib resection were symptom-free at a mean follow-up of 3.1 years [39]. By contrast, only 64% of the patients were symptom-free if thrombolysis failed to recanalize the vein.
Success rates of thrombolysis have been reported to range from 62 to 84% [41–43]. In our experience, lytic success exceeds 90%. Factors that adversely affect success are time to treatment and clot burden. The technique is more effective when given within 1 week of the onset of symptoms, which results in nearly uniform success. In a series from the University of Rochester (NY, USA), 26 patients underwent lysis with a mean time of 5.5 days after the onset of symptoms [42]. Long-segment occlusions (>5 cm) are correlated with low success rates, reported between 22 and 25%, often because of the chronicity of the disease [22,44].
Catheter-directed thrombolysis usually requires intensive care unit monitoring, regular blood tests and repeated venography to evaluate therapeutic efficacy. Although median times for complete thrombus resolution have been reported to be 24–48 h, we have obtained success in less than half of that time using pharmacomechanical techniques [45,46]. Moreover, the cost of thrombolysis followed by first rib resection can be considerably higher than managing the patient with anticoagulation alone. Hemorrhage is a recognized complication of thrombolysis [47,48]. To reduce hemorrhage risk and treatment duration, newer thrombolytic techniques have evolved, such as pharmacomechanical thrombectomy (PMT), which combines mechanical clot disruption and pharmacological thrombolysis within an isolated venous segment [48,49]. Patients treated with PMT often do not require intensive care unit monitoring and the systemic effects of lytic agents are reduced or eliminated. Implementing a strategy of thrombus removal using pharmacomechanical techniques, we have found that the acute thrombus can be uniformly eliminated in less than 24 h. A recent systematic review of PMT demonstrated a good safety profile, with no reported procedure-related deaths or strokes and <1% incidence of symptomatic pulmonary embolism [50]. Moreover, younger patients with acute limb compromise, such as patients with Paget–Schroetter syndrome, appear most likely to derive benefit from this technique [51].
The most commonly used PMT devices are the AngioJet® system (Medrad Inc., PA, USA), which uses a pulse-spray technique, the Trellis™ catheter (Covidien, CA, USA) and the EKOS Endowave™ system (EKOS Corporation, WA, USA). The double-balloon Trellis catheter isolates the segment of thrombosed vein using proximal and distal compliant balloons, thereby localizing the region of thrombolysis. Once the agent is infused into the targeted segment, the catheter assumes a sinusoidal configuration and oscillates at high frequency to disrupt the clot. The dissolved clot and lytic agent are subsequently aspirated. According to a recent meta-analysis, compared with CDT, the Trellis successfully lyses more clot (93 vs 79%) in a significantly shorter time [49].
The Trellis catheter system has advantages when compared with other PMT devices. It appears to have the lowest systemic thrombolytic activity, resulting in a reduced risk of hemorrhagic complications [52]. Postoperative hematoma or intrathoracic bleeding after first rib decompression is 8–17% [53,54]. It may also reduce the risk of distal embolic complications through its use of distal balloon occlusion and liquefied thrombus aspiration [55].
The EKOS device contains multiple ultrasound transducers, which emit high-frequency, low-energy ultrasound energy that thins the fibrin component of thrombus, exposing plasminogen receptors sites for more successful transportation of thrombolytic agents within target thrombus [56]. Theoretically, it has a lower potential for endothelial damage compared with rotational thrombectomy devices, but requires longer treatment times, with a mean of 22 h reported [56,57].
Surgical decompression
Thrombosis is the resultant consequence of an underlying chronic condition, which is generally extrinsic compression causing vein trauma at the costoclavicular junction, which is often associated with inadequate first rib collateral drainage. Thrombolysis treats the acute thrombotic complication of the chronic problem and restores venous patency. The subsequent venogram often shows residual extrinsic compression from a structural abnormality. It has been shown that if the anatomic abnormality is not corrected, rethrombosis has been reported in as many as a third of the patients [58–60]. In a systematic review of 11 series of 262 patients with upper extremity deep vein thrombosis treated by thrombolysis alone, 62 patients (24%) had residual symptoms and 18 (7%) had rethrombosis [61]. Consequently, surgical decompression of the thoracic outlet has become accepted as a fundamental component of treatment algorithms for patients with venous TOS. However, differences in opinions exist regarding the method, timing or even necessity of decompression.
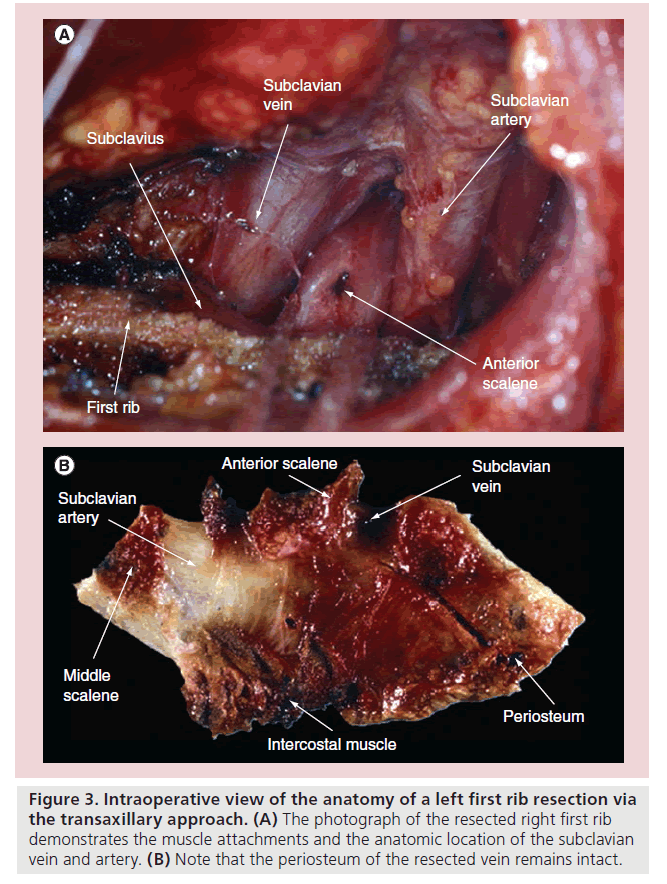
Early proponents of surgical decompression, Adams et al. reported that decompression of the subclavian vein resulted in durable relief of symptoms [62]. In 1966, Roos reported the first transaxillary rib resection, which, when properly performed, effectively decompresses the thoracic outlet and is preferred by the authors (Figure 3) [63]. Molina first described the pure infraclavicular approach for first rib resection [64]. Advantages of this approach include the clear exposure of the anterior portion of the first rib where it causes compression of the subclavian vein, and its excellent cosmesis. The drawback is the perceived technical difficulty in those inexperienced with the technique, which leads to the possibility of complications such as hemo-pneumothorax, arterial and vein injury, and long thoracic nerve injury. However, in experienced hands, the results are favorable in 85–95% of the patients [22,65,66].
Figure 3: Intraoperative view of the anatomy of a left first rib resection via the transaxillary approach. (A) The photograph of the resected right first rib demonstrates the muscle attachments and the anatomic location of the subclavian vein and artery. (B) Note that the periosteum of the resected vein remains intact.
An important technical point of the procedure is venolysis, which frees the vein circumferentially during the procedure so that it may respond to subsequent venoplasty [1]. Another technical point recommended by authors is the transection of the intercostobrachial cutaneous nerve, which is usually situated in the middle of the operative field. Stretching of this nerve to gain exposure can result in a prolonged severe burning pain of the underarm. Transection result in numbness in the same area, which is usually asymptomatic. Molina reported excellent results in 114 patients with an infraclavicular-only approach and routine vein patch angioplasty, demonstrating 100% patency in a mean follow-up of 5.2 years [64].
An alternative approach for decompression is the supraclavicular approach with or without an infraclavicular incision [53]. The supraclavicular approach permits wide exposure of the subclavian vein and is, therefore, very helpful in vascular reconstruction. It offers a chance for a definitive treatment regardless of the interval between initial diagnosis and referral, previous treatment, or adverse findings in venography [2,67,68]. Alternatively, other authors claim that the first rib removal via the supraclavicular/ infraclavicular incision does not offer any advantage [53]. Other more aggressive surgical approaches, such as sternal disarticulation with first rib resection [69,70] or medial claviculectomy [71], offer good exposure; however, the increased morbidity associated with these procedures has rendered them to be of historic interest only.
The majority would agree that CDT followed by thoracic outlet decompression is the preferred approach when symptoms persist after successful lysis. Although there are no randomized studies to clarify this issue, the results of most observational reports of thrombolysis followed by surgical decompression have resulted in generally favorable outcomes. In 1993, Machleder first described the algorithm of early CDT and first rib resection, reporting favorable results [39]. In 2007, Molina et al. reported 100% patency and no significant residual symptoms 5 years after CDT and first rib resection had been performed in patients with effort thrombosis [38]. They subsequently reported 100% patency over a 23-year period in 126 patients with early CDT and immediate surgical decompression [37]. Similarly, Stone et al. reported a patency rate of 100% at 1 year and 94% at 5 years in patients with early (within 12 days of symptom onset) CDT followed by immediate surgical decompression [30]. The largest retrospective study of patients with effort thrombosis, including 312 extremities over a 30-year experience, was published by Urschel and Razzuk [19]. The best results were in patients treated within 6 weeks of thrombosis using CDT followed by first rib resection. In patients who remained symptomatic and were treated 6 weeks or more after onset of symptoms, no vein could be completely opened with CDT and most patients remained symptomatic despite the first rib resection. In the same series, 35 patients were treated with anticoagulation alone, 26 experienced symptom recurrence after resuming normal work activities and 21 had long-term sequelae despite first rib resection. This observation highlights the importance of eliminating the acute thrombus.
Many authors suggest that surgical decompression is preferred to prevent recurrence. The timing of surgery remains open to opinion. The theoretical concern of increased thrombogenetic activity of venous endothelium after a thrombotic episode [72] and the perceived risks of operation with the need for systemic anticoagulation so soon after CDT led Machleder to propose delaying first rib resection until 3 months after thrombolysis [39]. However, this operative delay is associated with a 10% or more interval rethrombosis rate [39]. The author also suggests that only patients with persistent symptoms associated with venous obstruction would be suitable for surgery.
Angle et al. and Lee et al. independently compared the results of early and late surgical decompression [72,73]. They both concluded that early lysis followed by immediate decompression provides better overall clinical results than delayed decompression. Moreover, the experience of Urschel and Razzuk of early thrombolysis (within 6 weeks of the onset of symptoms) followed by first rib resection, generally performed the next day, appeared to result in favorable outcomes [19]. Of the 199 patients treated with this approach, 189 (95%) had excellent or good results and only two had poor results.
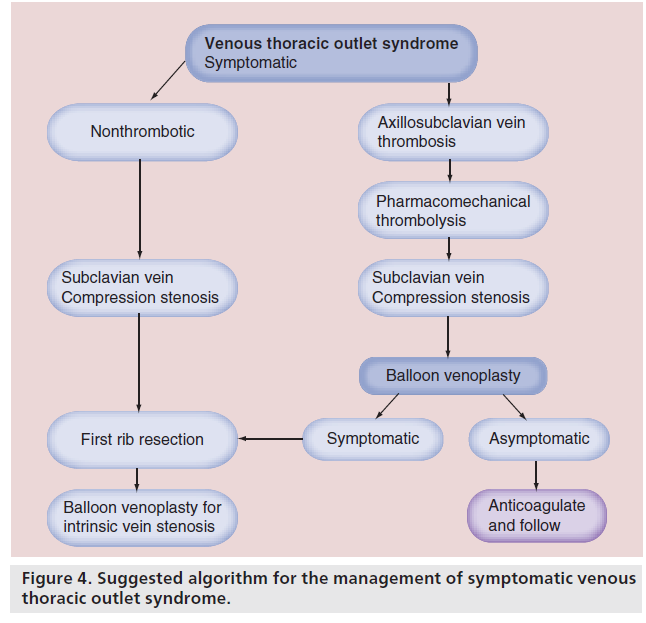
An alternative to uniform surgical decompression following thrombolysis is presented by Johansen, who described a series of 50 patients with primary effort thrombosis who underwent thrombolysis followed by anticoagulation and observation [74]. At a mean follow-up of 57 months, they found 82% of the cohort were entirely asymptomatic and 10% had only mild symptoms. However, the occlusion rate was found to be 18% and the incidence of patients with stenosis greater than 50% was 24%. The observations of Johansen [74], Sajid et al. [61], as well as our own clinical experience with these patients, have led us to adopt essentially uniform recommendations for CDT of acute axillosubclavian vein thrombosis. Once the acute thrombus is eliminated, and if the patient is asymptomatic, anticoagulation alone is recommended. If symptoms persist, we suggest first rib resection (Figure 4).
Management of residual intrinsic vein lesions
Surgical decompression does not correct the intrinsic vein defect that is a consequence of the chronic compression at the costoclavicular junction. Despite successful thrombolysis and surgical decompression, significant residual vein stenosis may persist [38,54], reducing venous return and increasing the risk of recurrent thrombosis [75]. The most appropriate management of residual lesions remains controversial. Options include anticoagulation alone, operative venous reconstruction (patch angioplasty, open venolysis or venous bypass) at the time of surgical decompression, or postoperative percutaneous transluminal angioplasty (PTA) with or without stenting. The proponents of anticoagulation alone argue that the failure rate of angioplasty or stenting is high and the procedurerelated endothelial damage is the major factor in rethrombosis. Proponents of surgical venolysis and decompression argue that remodeling of the lesion is expected to take place [66].
The role of PTA or stenting is controversial. The majority of authors agree that PTA with stenting should not be performed following successful thrombolysis if both first rib and clavicle remain to produce extrinsic compression [67]. If stents are placed without decompression of the thoracic outlet, they will be compressed between the clavicle and the first rib, which can lead to crushing of the stent, stent fracture and subsequent rethrombosis [76,77]. PTA is appropriate after lysis and surgical decompression of the thoracic outlet if a residual stenosis is present. Even then, there is lack of consensus about the timing of postoperative PTA, with recommendations ranging from the day of the procedure (concomitant surgical decompression and PTA) to 1 month postprocedure [39,67,72,77].
It would appear that a flow-limiting vein lesion in combination with the local surgical trauma occurring during decompression create a prothrombotic environment in the early postoperative period. Episodes of recurrent thrombosis have occurred in patients during the 3-day waiting period before venography and PTA of a possible residual stenosis [77]. By contrast, series of patients undergoing open surgical decompression combined with intraoperative venography and PTA show promising results. Schneider et al. studied 25 consecutive patients with acute effort thrombosis who underwent intraoperative venography after surgical decompression [77]. Residual intrinsic stenosis was identified in 16 patients (64%). All 16 patients underwent PTA with 100% technical success. The 1-year primary and secondary patency rates were 92 and 96%, respectively. It would appear that PTA has similar results to the more invasive and technically demanding open reconstruction, at least in the short term [2,38,39].
Despite the good outcomes of PTA in patients with intrinsic residual stenosis after surgical decompression, stenting does not seem to offer the same good results. Contrary to other vein beds, such as the iliac veins, it appears that PTA is more effective than stenting. Kreienberg et al. reported a series of 23 patients who underwent either balloon venoplasty or stenting immediately after surgical decompression [54]. Patency was 100% at 4 years in the nine patients undergoing venoplasty alone, but only 64% at 3.5 years in the 14 patients who were treated with stents. Other studies report high rates of failure of axillosubclavian vein stents, most likely due to the mobility of the shoulder joint and the axillary and subclavian veins [78,79]. The lack of randomized trials makes it difficult to determine the precise role of balloons and stents in the management of intrinsic vein stenoses. Moreover, the long-term durability of subclavian vein stents remains to be clarified. While these studies suggest that balloon venoplasty alone can have a favorable outcome in patients with residual stenosis following surgical decompression, and stents may have a worse prognosis than balloon angioplasty alone, one must be cautious about making such conclusions because stents may have been used to salvage an unsuccessful balloon venoplasty.
Another important question in patients with intrinsic residual lesions is the role of operative venous reconstruction. Some propose an aggressive approach performing venous reconstruction when external venolysis is unsuccessful, often with a temporary arteriovenous fistula [2,38,80,81]. Others advocate a less aggressive approach, reserving reconstruction in patients who have persistent and significant symptoms despite surgical decompression, external venolysis and balloon angioplasty [1]. The patency rate in series with less aggressive management was 94% [1].
Surgical techniques that have been used to treat proximal subclavian stenoses include patch angioplasty [80,81], vein bypass [80] and jugular vein turndown [82]. Patch angioplasty is suitable for short residual stenoses. For longer lesions, finding a suitably large vein to replace the axillosubclavian segment is often problematic [83]. Regarding the best approach for performing a venous reconstruction, some recommend that proximal claviculectomy permits the best exposure [1,71]. However, others claim that one can achieve the same good results by leaving the clavicle intact and avoiding the significant morbidity of painful arm function that proximal claviculectomy can cause [81,84].
Venous reconstruction can be valuable in a small subset of symptomatic patients with effort thrombosis whose veins remain occluded after attempting thrombolysis. An algorithm proposed by Illig and Doyle suggests decision-making based on the severity of the symptoms [1]. If the patient has mild or no symptoms, first rib resection is proposed. These patients can usually recanalize their occluded vein segment or develop a substantial collateral network [85]. By contrast, if symptoms are severe, vascular reconstruction should be performed, usually by jugular vein turndown because of the cord-like residual vein, which is not otherwise reconstructable. All agree that these patients should be anticoagulated. Owing to the low incidence of hypercoagulability, most agree that anticoagulation can be temporary, usually for 3–6 months.
Suggested treatment algorithm
We suggest a treatment algorithm for patients with venous TOS, based on our experience and the existing literature (Figure 4). We believe that all the patients with symptoms of effort thrombosis should undergo diagnostic venography in preparation for CDT. Successful thrombolysis of acute axillosubclavian vein thrombosis is generally very high. As with other catheter-based lytic procedures, guidewire passage through the thrombosed venous segment predicts success. In addition, a short duration of symptoms suggests higher acuity, whereas a long duration of symptoms suggests chronicity, which diminishes lytic success. Our preferred thrombolytic technique incorporates pharmacomechanical techniques using the Trellis device. Following thrombolysis, one of three scenarios is usually present:
• Complete recanalization of the vein, without residual stenosis (unusual);
• Partial recanalization, with residual intrinsic defect (most patients);
• Unsuccessful recanalization with the vein remaining occluded (not often).
However, even patients with a tight residual proximal subclavian vein stenosis or with segmental occlusion generally enjoy significant symptomatic improvement resulting from lysis of acute thrombus, which was occluding the venous drainage provided by their first rib collaterals. If patients are asymptomatic or minimally symptomatic following CDT, extended anticoagulation is recommended. Even in the presence of a residual stenosis or hyperextension-produced subclavian vein compression, only 15–20% will develop recurrent thrombosis. If recurrent thrombosis occurs, we will perform repeat CDT and then recommend first rib resection with correction of the intrinsic venous lesion. Otherwise, it appears that there is little to be gained by the remaining 80% of patients in having their first rib resected.
On the other hand, for patients who remain symptomatic after CDT because of residual subclavian vein stenosis and inadequate first rib collateral venous drainage, first rib resection with correction of the venous stenosis, usually with balloon venoplasty, is recommended. In a small percentage of patients, a venous stent may be required. Athletes who plan to return to their athletic activities will uniformly require first rib resection with correction of their venous stenosis to provide the necessary venous drainage at the time of increased arterial inflow to their upper extremity.
When surgical decompression is required, it is performed soon after thrombolysis as we believe there is no advantage in delaying the procedure. Since the indication for first rib resection is symptomatic venous outflow obstruction, delaying the procedure only prolongs the patients’ symptoms and exposes them to the risk of rethrombosis.
Conclusion
Venous TOS is generally a problem of young, active individuals, which, if left untreated, can adversely affect their quality of life. Our current clinical practice is based on single-center studies and our own clinical experience. Unfortunately, randomized data are not available to answer many of the important questions and, therefore, anticoagulation so far remains the gold standard. However, without such guidance, the proposed algorithm suggests a stepwise treatment approach that eliminates the acute thrombus, allowing for evaluation of the patients’ venous drainage. If it is fine, nothing more but anticoagulation is recommended and most will do well over the long term. If patients remain symptomatic after lysis of their acute clot, first rib resection followed by correction of their underlying venous stenosis is recommended.
Future perspective
Treatment of venous TOS, like most other diseases today, should be tailored to the patient’s needs. Some patients with thrombotic venous TOS will be well treated with anticoagulation alone; however, they will be those with a small burden of thrombus or nonocclusive thrombus. Patients with multisegment venous occlusion are those who are most symptomatic and will benefit from a strategy of thrombus removal. The next question is how best to treat an underlying venous lesion. We will need to properly select patients for thoracic outlet decompression and venous intervention, as it is clear that not all patients require these procedures, which have potential complications. We believe ongoing patient symptoms will become an important indicator for intervention for both thrombotic and nonthrombotic TOS. Of course, advances in catheter technology and oral anticoagulation continue, which will further improve our ability to offer effective and safe patient care.
Executive summary
• Venous thoracic outlet syndrome (TOS) represents approximately 5% of all cases of TOS.
• Venous TOS occurs most frequently in the right arm and many patients have a history of vigorous exercise of the involved extremity.
• The exact mechanism of venous injury associated with TOS is not clearly understood.
• Swelling, cyanosis and pain of the extremity are typical presenting symptoms.
• Venography is the diagnostic tool of choice.
• Catheter-directed thrombolytic therapy is the standard treatment of acute axillosubclavian thrombus.
• Surgical decompression (first rib resection) is often performed after successful lysis if symptoms persist, although its use is somewhat controversial.
• The management of residual vein stenoses is controversial, with options including anticoagulation, operative reconstruction or percutaneous transluminal angioplasty.
Financial & competing interests disclosure
AJ Comerota is a consultant for Covidien, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest
- Illig KA, Doyle AJ. A comprehensive review of Paget–Schroetter syndrome. J. Vasc. Surg. 51(6), 1538–1547 (2010).
- Melby SJ, Vedantham S, Narra VR et al. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget–Schroetter syndrome). J. Vasc. Surg. 47(4), 809–820 (2008).
- Paget J. Clinical Lectures and Essays. Longmans, Green & Co., London, UK (1875).
- von Schroetter L. Erkrankungen der Gefässe. In: Nathnagel Handbuch der Pathologie und Therapie. Holder, Vienna, Austria (1884).
- Hughes ES. Venous obstruction in the upper extremity. Br. J. Surg. 36(142), 155–163 (1948).
- Sanders RJ, Hammond SL. Subclavian vein obstruction without thrombosis. J. Vasc. Surg. 41(2), 285–290 (2005).
- Brooke BS, Freischlag JA. Contemporary management of thoracic outlet syndrome. Curr. Opin. Cardiol. 25(6), 535–540 (2010).
- Thakur S, Comerota AJ. Bilateral nonthrombotic subclavian vein obstruction causing upper extremity venous claudication. J. Vasc. Surg. 52(1), 208–211 (2010).
- Charrette EJ, Iyengar KS, Lynn RB, Challis TW. Symptomatic non-thrombotic subclavian vein obstruction. Surgical relief in six patients. Vasc. Surg. 7(4), 220–231 (1973).
- Lindblad B, Tengborn L, Bergqvist D. Deep vein thrombosis of the axillary-subclavian veins: epidemiologic data, effects of different types of treatment and late sequelae. Eur. J. Vasc. Surg. 2(3), 161–165 (1988).
- Prandoni P, Bernardi E. Upper extremity deep vein thrombosis. Curr. Opin. Pulm. Med. 5(4), 222–226 (1999).
- Sternbach Y, Green RM. Endovascular and surgical managment of acute axillary-subclavian venous thrombosis. In: Handbook of Venous Disease (2nd Edition). Gloviczki P, Yao J (Eds). Hodder Arnold, London, UK, 209–213 (2001).
- Horattas MC, Wright DJ, Fenton AH et al. Changing concepts of deep venous thrombosis of the upper extremity – report of a series and review of the literature. Surgery 104(3), 561–567 (1988).
- Gray, H. Osteology. In: Gray’s Anatomy. Williams PL, Warwick R, Dyon M, Bannister LH (Eds). Churchill Livingstone, London, UK, 267–458 (1989).
- Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet: an analysis of 200 consecutive cases. J. Vasc. Surg. 16(4), 534–542 (1992).
- Adams JT, DeWeese JA, Mahoney EB, Rob CG. Intermittent subclavian vein obstruction without thrombosis. Surgery 68, 147–165 (1968).
- Falconer MA, Weddell GL. Costoclavicular compression of subclavian arery and vein. Lancet 242, 539–543 (1943).
- Sampson JJ, Saunders JB, Capp CS. Compression of the subclavian vein by first rib and clavicle. Am. Heart J. 19, 292–315 (1940).
- Urschel HC Jr, Razzuk MA. Paget–Schroetter syndrome: what is the best management? Ann. Thorac. Surg. 69(6), 1663–1668 (2000).
- Aziz S, Straehley CJ, Whelan TJ Jr. Effort-related axillosubclavian vein thrombosis. A new theory of pathogenesis and a plea for direct surgical intervention. Am. J. Surg. 152(1), 57–61 (1986).
- Flinterman LE, van der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J. Thromb.Haemost. 6(8), 1262–1266 (2008).
- Thompson RW, Schneider PA, Nelken NA, Skioldebrand CG, Stoney RJ. Circumferential venolysis and paraclavicular thoracic outlet decompression for ‘effort thrombosis’ of the subclavian vein. J. Vasc. Surg. 16(5), 723–732 (1992).
- Joffe HV, Goldhaber SZ. Upper-extremity deep vein thrombosis. Circulation 106(14), 1874–1880 (2002).
- Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J. Vasc. Surg. 46(3), 601–604 (2007).
- Tsekouras N, Comerota AJ. Phlegmasia cerulea dolens. In: Color Atlas and Synopsis of Vascular Disease. Dean SM, Satiani B (Eds).McGraw-Hill Education, NY, USA, 263–268 (2013).
- Thompson RW. Comprehensive management of subclavian vein effort thrombosis. Semin. Intervent. Radiol. 29(1), 44–51 (2012).
- Sharafuddin MJ, Sun S, Hoballah JJ. Endovascular management of venous thrombotic diseases of the upper torso and extremities. J. Vasc. Interv. Radiol. 13(10), 975–990 (2002).
- Ho VB, van Geertruyden PH, Yucel EK et al. ACR appropriateness criteria(®) suspected lower extremity deep vein thrombosis. J. Am. Coll. Radiol. 8(6), 383–387 (2011).
- Hingorani A, Ascher E, Lorenson E et al. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J. Vasc. Surg. 26(5), 853–860 (1997).
- Stone DH, Scali ST, Bjerk AA et al. Aggressive treatment of idiopathic axillo-subclavian vein thrombosis provides excellent long-term function. J. Vasc. Surg. 52(1), 127–131 (2010).
- Green RM, Rosen R. The management of axillo-subclavian venous thrombosis in the setting of thoracic outlet syndrome. In: Handbook of Venous Disorders (3rd Edition). Gloviczki P (Ed.) Hodder Arnold,London, UK, 292–298 (2008).
- Guzzo JL, Chang K, Demos J, Black JH, Freischlag JA. Preoperative thrombolysis and venoplasty affords no benefit in patency following first rib resection and scalenectomy for subacute and chronic subclavian vein thrombosis. J. Vasc. Surg. 52(3), 658–662 (2010).
- Adams JT, DeWeese JA. ‘Effort’ thrombosis of the axillary and subclavian veins. J. Trauma 11(11), 923–930 (1971).
- Heron E, Lozinguez O, Emmerich J, Laurian C, Fiessinger JN. Long-term sequelae of spontaneous axillary-subclavian venous thrombosis. Ann. Intern. Med. 131(7), 510–513 (1999).
- AbuRahma AF, Sadler D, Stuart P, Khan MZ, Boland JP. Conventional versus thrombolytic therapy in spontaneous (effort) axillary-subclavian vein thrombosis. Am. J. Surg. 161(4), 459–465 (1991).
- Monreal M, Lafoz E, Ruiz J, Valls R, Alastrue A. Upper-extremity deep venous thrombosis and pulmonary embolism. A prospective study. Chest 99(2), 280–283 (1991).
- Molina JE, Hunter DW, Dietz CA. Protocols for Paget–Schroetter syndrome and late treatment of chronic subclavian vein obstruction. Ann. Thorac. Surg. 87(2), 416–422 (2009).
- Molina JE, Hunter DW, Dietz CA. Paget–Schroetter syndrome treated with thrombolytics and immediate surgery. J. Vasc. Surg. 45(2), 328–334 (2007).
- Machleder HI. Evaluation of a new treatment strategy for Paget–Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J. Vasc. Surg. 17(2), 305–315 (1993).
- Sanders RJ, Hammond SL. Venous thoracic outlet syndrome. Hand Clin. 20(1), 113–118 (2004).
- Beygui RE, Olcott C, Dalman RL. Subclavian vein thrombosis: outcome analysis based on etiology and modality of treatment. Ann. Vasc. Surg. 11(3), 247–255 (1997).
- Doyle A, Wolford HY, Davies MG et al. Management of effort thrombosis of the subclavian vein: today’s treatment. Ann. Vasc. Surg. 21(6), 723–729 (2007).
- Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C. Long-term thrombotic recurrence after nonoperative management of Paget–Schroetter syndrome. J. Vasc. Surg. 43(6), 1236–1243 (2006).
- Molina JE. Need for emergency treatment in subclavian vein effort thrombosis. J. Am. Coll. Surg. 181(5), 414–420 (1995).
- Schneider DB, Curry TK, Eichler CM, Messina LM, Gordon RL, Kerlan RK. Percutaneous mechanical thrombectomy for the management of venous thoracic outlet syndrome. J. Endovasc. Ther. 10(2), 336–340 (2003).
- Shah AD, Bajakian DR, Olin JW, Lookstein RA. Power-pulse spray thrombectomy for treatment of Paget–Schroetter syndrome. Am. J. Roentgenol. 188(5), 1215–1217 (2007).
- Vik A, Holme PA, Singh K et al. Catheter-directed thrombolysis for treatment of deep venous thrombosis in the upper extremities. Cardiovasc. Intervent. Radiol. 32(5), 980–987 (2009).
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology 211(1), 39–49 (1999).
- Hilleman DE, Razavi MK. Clinical and economic evaluation of the Trellis-8 infusion catheter for deep vein thrombosis. J. Vasc. Interv. Radiol. 19(3), 377–383 (2008).
- Karthikesalingam A, Young EL, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. A systematic review of percutaneous mechanical thrombectomy in the treatment of deep venous thrombosis. Eur. J. Vasc. Endovasc. Surg. 41(4), 554–565 (2011).
- Gogalniceanu P, Johnston CJ, Khalid U et al. Indications for thormbolysis in deep venous thrombosis. Eur. J. Vasc. Endovasc. Surg. 38(2), 192–198 (2009).
- O’Sullivan GJ, Lohan DG, Gough N, Cronin CG, Kee ST. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J. Vasc. Interv. Radiol. 18(6), 715–724 (2007).
- Divi V, Proctor MC, Axelrod DA, Greenfield LJ. Thoracic outlet decompression for subclavian vein thrombosis: experience in 71 patients. Arch. Surg. 140(1), 54–57 (2005).
- Kreienberg PB, Chang BB, Darling RC et al. Long-term results in patients treated with thrombolysis, thoracic inlet decompression, and subclavian vein stenting for Paget–Schroetter syndrome. J. Vasc. Surg. 33(2 Suppl.), S100–S105 (2001).
- Tsai J, Georgiades CS, Hong K, Kim HS. Presumed pulmonary embolism following power-pulse spray thrombectomy of upper extremity venous thrombosis. Cardiovasc. Intervent. Radiol. 29(4), 678–680 (2006).
- Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med. Biol. 21(3), 419–424 (1995).
- Parikh S, Motarjeme A, McNamara T et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J. Vasc. Interv. Radiol. 19(4), 521–528 (2008).
- Druy EM, Trout HH 3rd, Giordano JM, Hix WR. Lytic therapy in the treatment of axillary and subclavian vein thrombosis. Vasc. Surg. 2(6), 821–827 (1985).
- Machleder HI. Upper extremity venous occlusion. In: Current Therapy in Vascular Surgery. Ernst CB, Stanley JC (Eds). Mosby,St Louis, MO, USA, 958–963 (1995).
- Strange-Vognsen HH, Hauch O, Andersen J, Struckmann J. Resection of the first rib, following deep arm vein thrombolysis in patients with thoracic outlet syndrome. Cardiovasc. Surg. (Torino) 30(3), 430–433(1989).
- Sajid MS, Ahmed N, Desai M, Baker D, Hamilton G. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 118(1), 10–18 (2007).
- Adams JT, McEvoy RK, DeWeese JA. Primary deep venous thrombosis of upper extremity. Arch. Surg. 91, 29–41 (1965).
- Roos DB. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann. Surg. 163(3), 354–358 (1966).
- Molina JE. Surgery for effort thrombosis of the subclavian vein. J. Thorac. Cardiovasc. Surg. 103(2), 341–346 (1992).
- Urschel HC Jr, Razzuk MA. Neurovascular compression in the thoracic outlet: changing management over 50 years. Ann. Surg. 228(4), 609–617 (1998).
- Freischlag J. Venous thoracic outlet syndrome: transaxillary approach. Op. Tech. Gen. Surg. 10(3), 122–130 (2008).
- Azakie A, McElhinney DB, Thompson RW, Raven RB, Messina LM, Stoney RJ. Surgical management of subclavian-vein effort thormbosis as a result of thoracic outlet compression. J. Vasc. Surg. 28(5), 777–786 (1998).
- Melby SJ, Thrompson RW. Supraclavicular (paraclavicular) approach for thoracic outlet syndrome. In: Operative Vascular Surgery in the Endovascular Era. Pearce WH,Matsumura JS, Yao JST (Eds). Greenwood Academic, Evanston, IL, USA, 434–445 (2008).
- Molina JE. Approach to the confluence of the subclavian and internal jugular veins without claviculectomy. Semin. Vasc. Surg. 13(1), 10–19 (2000).
- Molina JE. Treatment of chronic obstruction of the axillary, subclavian, and innominate veins. Int. J. Angiol. 8, 87–90 (1999).
- Green RM, Waldman D, Ouriel K, Riggs P, DeWeese JA. Claviculectomy for subclavian venous repair: long-term functional results. Vasc. Surg. 32(2), 315–321 (2000).
- Angle N, Gelabert HA, Farooq MM et al. Safety and efficacy of early surgical decompression of the thoracic outlet for Paget–Schroetter syndrome. Ann. Vasc. Surg. 15(1), 37–42 (2001).
- Lee MC, Grassi CJ, Belkin M, Mannick JA, Whittemore AD, Donaldson MC. Early operative intervention after thrombolytic therapy for primary subclavian vein thrombosis: an effective treatment approach. J. Vasc. Surg. 27(6), 1101–1107 (1998).
- Johansen K. Does axillosubclavian vein thrombosis oblige first rib resection. Presented at: Western Surgical Association. Santa Fe, NM, USA, 9 November 2008.
- Machleder HI. Thrombolytic therapy and surgery for primary axillosubclavian vein thrombosis: current approach. Semin. Vasc. Surg. 9(1), 46–49 (1996).
- Urschel HC Jr, Patel AN. Paget–Schroetter syndrome therapy: failure of intravenous stents. Ann. Thorac. Surg. 75(6), 1693–1696 (2003).
- Schneider DB, Dimuzio PJ, Martin ND et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J. Vasc. Surg. 40(4), 599–603 (2004).
- Maintz D, Landwehr P, Gawenda M, Lackner K. Failure of Wallstents in the subclavian vein due to stent damage. Clin. Imag. 25(2), 133–137 (2001).
- Vesely TM, Hovsepian DM, Pilgram TK, Coyne DW, Shenoy S. Upper extremity central venous obstruction in hemodialysis patients: treatment with Wallstents. Radiology 204(2), 343–348 (1997).
- Thompson RW, Petrinec D, Toursarkissian B. Surgical treatment of thoracic outlet compression syndromes. II. Supraclavicular exploration and vascular reconstruction. Ann. Vasc. Surg. 11(4), 442–451 (1997).
- Thompson RW. Venous thoracic outlet syndrome: paraclavicular approach. Op. Tech. Gen. Surg. 10 (3), 113–121 (2008).
- Puskas JD, Gertler JP. Internal jugular to axillary vein bypass for subclavian vein thrombosis in the setting of brachial arteriovenous fistula. J. Vasc. Surg. 19(5), 939–942 (1994).
- Spivack A, Troutman D, Dougherty M, Calligaro K. Changing strategies to treat venous thrombotic occlusions of the upper and lower extremities secondary to compressive phenomena. Vasc. Endovasc. Surg. 47(4), 274–277 (2013).
- Yu SH, Dilley RB. Internal jugular vein turndown for subclavian vein occlusion. Op. Tech. Gen. Surg. 10(3), 149–153 (2008).
- de León R, Chang DC, Busse C, Call D, Freischlag JA. First rib resection and scalenectomy for chronically occluded subclavian veins: what does it really do? Ann. Vasc. Surg. 22(3), 395–401 (2008).
•• Reviews the strategy of thrombus removal and thoracic outlet decompression in patients with Paget–Schroetter syndrome. In addition to discussing the overall treatment algorithm in detail, the authors attempt to illustrate the controversies that remain and the clinical and basic research directions that need to be pursued. A discussion of existing controversies in the management of Paget–Schroetter syndrome is included.
•• Describes how effort thrombosis is distinct from other forms of deep vein thrombosis with respect to pathophysiology, clinical presentation and functional consequences. The author describes management options unique to venous thoracic outlet compression as well as the obstructed subclavian vein.
•• The authors review their experience with anticoagulation alone versus catheter-directed thrombolytic therapy for axillosubclavian venous thrombosis. The results indicate a greater success rate in patients who were treated with thrombolysis and a higher rate of persistent occlusion and post-thrombotic syndrome in patients treated with anticoagulation alone.
•• The authors contrast their experience of percutaneous angioplasty alone versus angioplasty and stenting following thrombolysis and first rib decompression for Paget–Schroetter syndrome. All patients with percutaneous angioplasty alone had patent stents at 4 years, whereas 50% of patients who were stented had occlusion at 3.5 years of follow-up.
•• The authors describe three cases of recurrent venous obstruction of the subclavian vein treated with WALLSTENTS® (Boston Scientific, MA, USA). All three patients developed recurrent thrombosis due to stent failure, with two stents fragmenting and one stent completely collapsing.