Device Evaluations - Interventional Cardiology (2012) Volume 4, Issue 1
Cotavance paclitaxel-eluting balloon: review of preclinical and clinical study data
- Corresponding Author:
- Nicolas Diehm
Swiss Cardiovascular Center
Clinical & Interventional Angiology, Inselspital
University Hospital Bern, Switzerland
E-mail: diehm@gmx.ch
Abstract
Keywords
drug-coated balloon, drug-eluting balloon, neointimal hyperplasia, restenosis, stent
Balloon angioplasty was introduced more than three decades ago and has since then matured significantly as the primary revascularization strategy for many patients with peripheral arterial disease (PAD). Driven by the pioneering spirit of vascular interventionalists and technical innovations of medical device industry partners, a variety of available devices has substantially expanded endovascular treatment options for PAD patients in recent years. Introduction of novel wire technology and re-entry catheters has facilitated interventional treatment of challenging long-segment and heavily calcified arterial obstructions. While technical success has thereby increased significantly, clinical outcomes subsequent to this minimal-invasive treatment option are still hampered by restenosis.
The incidence of restenosis after plain old balloon angioplasty (POBA) of lower limb arteries increases with decreasing vessel diameter. While it occurs rarely in patients undergoing endovascular iliac artery stent revascularization [1], it was shown to impact clinical outcomes in up to 60% after femoropopliteal stenting and in up to more than two-thirds of patients [2] after long-segment below-the-knee (BTK) angioplasty.
Overview of the market
It is estimated that PAD currently affects 27 million people in Europe and North America with 500 to 1000 new cases per million being diagnosed annually [3]. At present, more than 1.5 million endovascular revascularization procedures are carried out in the USA and in Europe with expected annual growth rates of 5% or more [101].
In the femoropopliteal segment, numerous attempts to increase vessel patency after POBA have included systemically applied medications and endovascular brachytherapy, as well as the introduction of stents [4–6]. Despite technical refinements in the latest Nitinol stent technology, restenosis occurs in approximately every third patient undergoing femoropopliteal stenting [4]. Initial clinical trials with drug-eluting stents (DES) have failed to indicate restenosis inhibition in the femoropopliteal segment [7]. Recently, the latest-generation highly flexible paclitaxel-eluting Nitinol stents were shown to significantly lower restenosis rates when compared with bare nitinol stents or POBA [8]. However, it has to be kept in mind that the femoropopliteal segment is a highly mobile vascular territory being subjected to a variety of movements during daily exercise. Thus, the long-term clinical impact of stent fractures after use of DES remains to be clarified.
Similarly, the introduction of coronary balloon-expandable DES has significantly improved patency rates after BTK angioplasty [9–11]. Of note, arterial lesion lengths in these studies were substantially shorter than those encountered in many patients with critical limb ischemia undergoing endovascular BTK treatment. Thus, the recently reported superiority of DES in BTK angioplasty applies to a very small subset of critical limb ischemia patients and the general applicability of these studies to everyday practice is limited. The advent of drug-eluting balloon (DEB) technology has fostered great hopes, especially for PAD patients in whom the use of a metal implant is undesirable, such as in very tiny or highly mobile arterial segments. The purpose of this article is to review preclinical and clinical studies of the Cotavance® Paclitaxel-coated Balloon Angioplasty Catheter for lower limb endovascular interventions.
Introduction to the device
Paclitaxel, a member of the drug category taxane, is commonly used in the treatment of a broad range of neoplasms. Paclitaxel is a diterpenoid that promotes the assembly of microtubules from tubulin dimers and stabilizes microtubules by preventing depolymerization, leading to the inhibition of cell division and migration. Cell culture experiments have shown that a short-term contact between vascular smooth cells and lipophilic taxane compounds may inhibit cell growth [12–14]. Preclinical studies with Paccocath® paclitaxel-coated balloon catheters in coronary and peripheral arteries in swine indicated that paclitaxel reduces neointimal proliferation even with short-term delivery of the drug to the vessel wall [15–17]. Additional porcine coronary studies revealed Paccocath balloons to be superior in reducing neointimal area compared with DES and, when paired with a bare-metal stent, allow a similar degree of endothelization as uncoated balloons, all with no thrombotic complications or other significant adverse events [18]. In summary, these animal studies demonstrated the safety and efficacy of this method as a lesion-specific alternative for drug transfer to the vessel wall.
Clinical profile & post-market findings
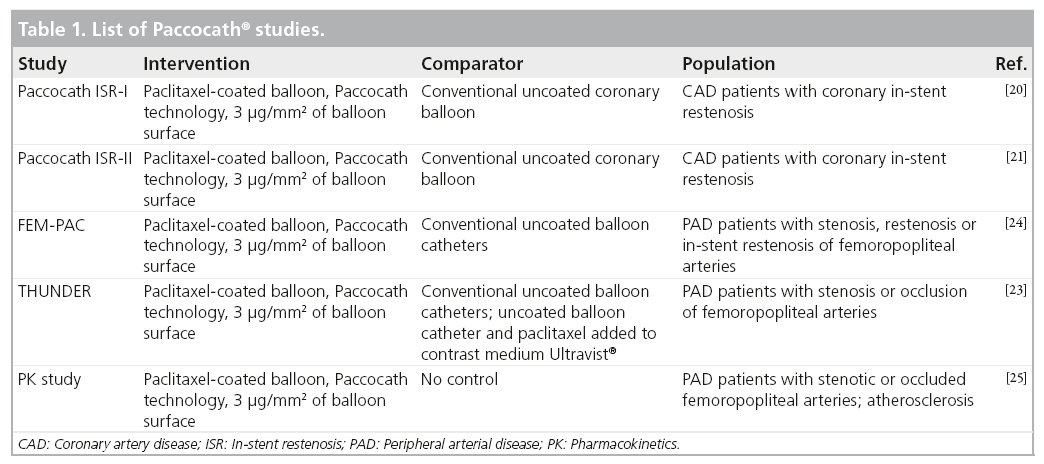
The clinical and angiographic utility of the Paccocath technology has been assessed in various clinical trials, both in coronary and lower limb arteries (Table 1).
In 2006 Scheller et al. presented the first results of paclitaxel-coated balloons for coronary in-stent restenosis treatment in humans, demonstrating a significant reduction of insegment late lumen loss and restenosis within the group of patients treated by DEB, compared with the group of patients treated by POBA [19]. Multiple studies on the clinical efficacy of the paclitaxel-coated balloon application for the reduction of restenosis in the coronary arteries have followed [19–21].
Within a multicenter study design, Tepe et al. randomly allocated 154 symptomatic patients with PAD into three treatment arms comparing paclitaxel-coated angioplasty balloons utilizing the Paccocath technology (a coating comprising of paclitaxel and an iopromide carrier), with POBA using paclitaxel-containing contrast material intra-arterially and with conventional POBA (THUNDER trial) [22]. The primary end point was late lumen loss. Secondary end points were technical success, 6-month restenosis rate as determined by intra-arterial angiography, change of PAD severity stage according to Rutherford [23], ankle-brachial index and arterial patency rate, as well as the need for repeated target lesion revascularization (TLR).
At the 6-month angiographic follow-up (128/154 [83%] of the patients), treatment with the Paccocath technology was associated with a significant reduction in late lumen loss in the control group (1.7 ± 1.8 mm) as compared with the group treated with paclitaxel-coated balloons (0.4 ± 1.2 mm; p < 0.001) and the group treated with paclitaxel in the contrast medium (2.2 ± 1.6 mm; p = 0.11).
Moreover, the angiographic restenosis rate was significantly lower among patients treated with Paccocath balloons compared with patients in the control group (17 vs 44%; p = 0.01). The TLR rate at 6 months was 20/54 (37%) in the control group, 2/48 (4%) in the group treated with paclitaxel-coated balloons (p < 0.001 vs control) and 15/52 (29%) in the group treated with paclitaxel in the contrast medium (p = 0.41 vs control). Regarding clinical improvement, the three study arms did not differ significantly from each other.
Werk et al. compared paclitaxel-coated balloon catheters with Paccocath technology with conventional POBA within the femoral paclitaxel pilot trial, a randomized study comprising 87 patients [23]. Inclusion criteria were occlusions or hemodynamically relevant stenosis, restenosis or in-stent restenosis of the femoropopliteal arteries. As in the THUNDER study, the primary end point was late lumen loss at 6 months. Secondary end points included binary restenosis, ankle brachial index, Rutherford class and the need for target lesion revascularization.
At the 6-month follow-up, angiography was performed in 31/45 (69%) of the coated balloon and 34/42 (81%) of the uncoated balloon patients. Late lumen loss was significantly lower in the Paccocath group compared with control patients (0.5 ± 1.1 vs 1.0 ± 1.1 mm, respectively; p = 0.031). Accordingly, compared with the control group, rates of binary restenosis (6/31 [19%] vs 16/34 [47%]) and TLR (3/45 [7%] vs 14/42 [33%]) were significantly lower in Paccocath patients. Finally, patients in the DEB group exhibited an improvement in Rutherford class (p = 0.045), but no difference in the improvement of hemodynamic workup was found.
A pharmacokinetic study was conducted to investigate plasma levels and catheter tolerability following angioplasty with paclitaxel-coated balloon catheters (devices were manufactured by MEDRAD Interventional®, USA) [24]. Fourteen patients with stenotic or occluded femoropopliteal arteries were included in the study. The maximum paclitaxel plasma concentration was reached immediately post intervention (0–25 min). The plasma concentration returned to the lower limit of values that are quantifiable at 24 h post-intervention in all patients. This indicates that there is no systemic bioavailability of paclitaxel ≥24 h following paclitaxel-coated balloon angioplasty. The patients were followed for 4 weeks after the intervention for safety. Overall, the paclitaxel-coated balloon catheter with Paccocath technology was well-tolerated and safe in patients with PAD.
Future Paccocath studies & preliminary DE B experiences in BTK arteries
The safety and efficacy of the Cotavance Paclita xel-coated Ba lloon Angioplast y Catheter with Paccocath technology will be assessed in several prospective randomized trials in patients with femoropopliteal de novo lesions (RIVER study) and in-stent restenoses (COPACABANA study). Moreover, the concept of atherectomy in adjunction with DEB angioplasty will be assessed in a randomized fashion in the DEFINITIVE AR study.
Data on the clinical utility of DEB in the BTK arteries is currently limited to a single-center nonrandomized registry assessing a different paclitaxel DEB device, utilizing urea as the carrier on the balloon. Schmidt et al. treated 104 patients with critical limb ischemia or severe claudication with BTK arterial involvement by DEB angioplasty [2]. Mean lesion length was 176 ± 88 mm. Angiographic restenosis at 3 months was 27.4% and usually occurred focally. In an earlier publication from the same center, 3-month angiographic restenosis rates following POBA of a comparable patient cohort were reported to be 69% [25]. Thus, based on these nonrandomized observations, restenosis rates subsequent to BTK angioplasty may be reduced using paclitaxel-coated balloons.
The EuroCanal study will randomize a total of 120 patients with critical limb ischemia and obstructions of the BTK arteries, to Cotavance DEB angioplasty versus POBA. This study started enrolment in September 2011. Further studies with various DEB devices are currently enrolling or are planned to investigate the angiographic and clinical utility of DEB in BTK arteries.
Device description
The Cotavance Paclitaxel-coated Balloon Angioplasty Catheter is a sterile, single-patient use, over-the-wire, percutaneous transluminal angioplasty balloon catheter (Figure 1). Cotavance catheters are designed for use with standard commercially available guidewires, introducers, inflation devices and syringes. The Cotavance DEB principle of operation is similar to other percutaneous transluminal angioplasty devices on the market. It works by placing the semi-compliant balloon within a blockage and inflating it with sufficient force for at least 60 s to dilate stenotic lesions.
The unique feature of the Cotavance paclitaxelcoated balloon catheter is an adherent drug coating on the balloon for inhibiting restenosis after angioplasty; the proprietary name for this drug coating is Paccocath technology. The coating is a blend of the contrast agent (Ultravist®) as a carrier and paclitaxel as the active medicinal substance for reducing neointimal proliferation subsequent to angioplasty. During balloon inflation, the coating is transferred to the arterial wall by contact. After deflation, the drug remains in the target arterial tissue to prevent neointimal hyperplasia and thereby inhibit restenosis.
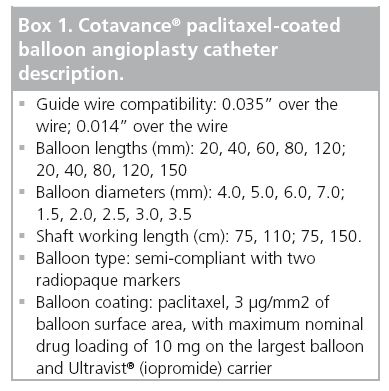
The balloon is CE-marked and commercially available on a 0.035” and a 0.014” platform and in two different shaft lengths (Box 1).
How the technology fits into the field of medical devices
Drug-coated balloons have a number of potential advantages over POBA and stent implantation for the treatment of PAD:
▪ Lower extremity arteries are subject to complex deformations during leg movement [26]. Therefore, the implantation of stents may be associated with stent fracture and restenosis [27]. Use of DEB allows for a local application of paclitaxel, subsequent to angioplasty, without the need for a permanent metal implant such as a stent;
▪An immediate paclitaxel release without the need for a polymer that can induce chronic inflammation and late thrombosis as observed in DES [28];
▪ Lower restenosis rates after angioplasty of femoropopliteal arteries compared with POBA.
POBA was shown to be associated with unacceptably high restenosis rates in the femoropopliteal and BTK arterial segment. The introduction of DES has substantially lowered restenosis rates in patients with comparatively short lesions of the femoropopliteal segment [8]. How DES compare to drug-coated balloons in femoropopliteal and BTK lesions remains to be determined.
DEB may have an important role as antirestenosis therapy in many patients with PAD and may be a very interesting treatment alternative when stents are undesirable, such as in long BTK or in small-diameter arteries.
Alternative devices
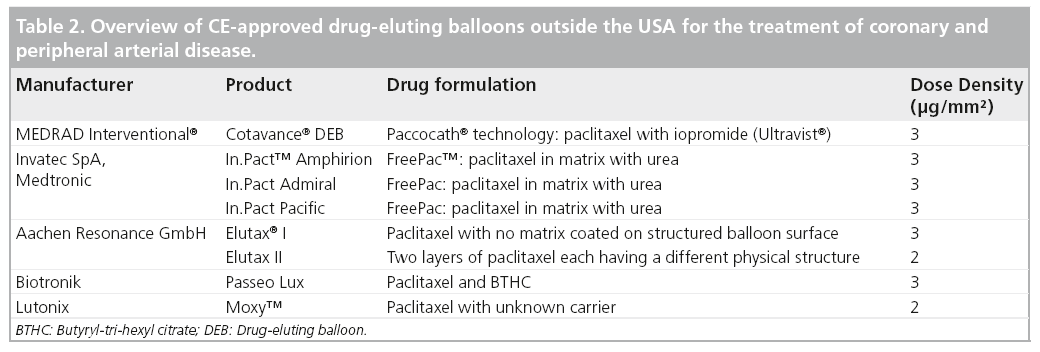
A variety of drug-coated balloons is currently commercially available (Table 2). These devices differ mainly with regard to the paclitaxel dose and carrier on the balloon surface. At present, positive data from randomized clinical studies are available for femoropopliteal revascularization with the Cotavance DEB, the IN.PACT™ DEB and the Moxy™ DEB. Of note, the largest body of published preclinical and clinical science to date has been obtained for the Cotavance DEB with Paccocath technology.
Conclusion
In conclusion, DEB technology has the potential to revolutionize antirestenosis therapy in lower limb endovascular intervention.
Among the currently commercially available devices which vary with regard to both the paclitaxel dose and the carrier, the Cotavance DEB is the one with the most clinical evidence in endovascular revascularization for PAD.
It has to be kept in mind however, that the currently published scientific scrutiny of this technology is limited to three randomized trials, with a total of 342 patients. Further studies are required to better define the benefit of DEB versus other promising treatment concepts, such as DES or absorbable stents in patients with PAD.
Future perspective
The Cotavance Paclitaxel-coated Balloon Angioplasty Catheter may potentially be of further advantage for several other endovascular therapies, such as renal artery angioplasty, angioplasty of arteriovenous access grafts and arterial obstructions caused by inflammatory disorders. Some of these potential indications have not been studied clinically.
Financial & competing interests disclosure
N Diehm is a paid consultant to MEDRAD, Inc. and principal investigator of the EuroCanal Study, a randomized multicenter trial investigating the use of the Cotavance® balloon catheter in infrapopliteal arteries. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Mechanism of action
▪ Suppression of neointimal hyperplasia by local administration of paclitaxel during balloon angioplasty.
Pharmacokinetics
▪ Absorption of paclitaxel, a lipophilic antiproliferative drug that is contained on the balloon beside a carrier (Iopromide).
Clinical efficacy
▪ Significant reduction in restenosis subsequent to endovascular femoropopliteal revascularization as compared with plain old balloon angioplasty (as shown in two randomized multicenter studies).
Safety & tolerability
▪ Equivalent tolerability and safety compared with an uncoated balloon.
Dosage & administration
▪ Paclitaxel 3 μg/mm2 of balloon surface.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Norgren L, Hiatt W, Dormandy J, Nehler M, Harris K, Fowkes F. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 45(Suppl. 1), S5–S67 (2007).
- Schmidt A, Piorkowski M, Werner M et al. First experience with drug-eluting balloons in infrapopliteal arteries restenosis rate and clinical outcome. J. Am. Coll. Cardiol. 58(11), 1105–1109 (2011).
- Belch J, Topol E, Agnelli G et al. Critical issues in peripheral arterial disease detection and management. A call to action. Arch. Intern. Med. 163, 884–892 (2003).
- Schillinger M, Minar E. Past, present and future of femoropopliteal stenting. J. Endovasc. Ther. 16(Suppl. 1), 147–152 (2009).
- Diehm N, Silvestro A, Do DD et al. Endovascular brachytherapy after femoropopliteal balloon angioplasty fails to show robust clinical benefit over time. J. Endovasc. Ther. 12(6), 723–730 (2005).
- Gallino A, Do DD, Alerci M et al. Effects of probucol versus aspirin and versus brachytherapy on restenosis after femoropopliteal angioplasty: the PAB randomized multicenter trial. J. Endovasc. Ther. 11(6), 595–604 (2004).
- Duda SH, Bosiers M, Lammer J et al. Sirolimus-eluting versus bare nitinol stent for obstructive superficial femoral artery disease: the SIROCCO II trial. J. Vasc. Interv. Radiol. 16(3), 331–338 (2005).
- Dake M. Zilver PTX trial: 2-year results. Presented at: The International Symposium on Endovascular Therapy. Miami, FL, USA, 15–20 January 2011.
- Rastan A, Tepe G, Krankenberg H et al. Sirolimus-eluting stents vs bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur. Heart J. 32(18), 2274–2281 (2011).
- Scheinert D. The Achilles Study. Presented at: The Leipzig Interventional Course. Leipzig, Germany, 19–22 January 2011.
- Bosiers M, Deloose K, Callaert J, Keirse K, Verbist J, Peeters P. Drug-eluting stents below the knee. J. Cardiovasc. Surg. 52(2), 231–234 (2011).
- Axel DI, Kunert W, Goggelmann C et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 96(2), 636–645 (1997).
- Scheller B, Speck U, Romeike B et al. Contrast media as carriers for local drug delivery. Successful inhibition of neointimal proliferation in the porcine coronary stent model. Eur. Heart J. 24(15), 1462–1467 (2003).
- Scheller B, Speck U, Schmitt A, Bohm M, Nickenig G. Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J. Am. Coll. Cardiol. 42(8), 1415–1420 (2003).
- Speck U, Scheller B, Abramjuk C et al. Neointima inhibition: comparison of effectiveness of non-stent-based local drug delivery and a drug-eluting stent in porcine coronary arteries. Radiology 240(2), 411–418 (2006).
- Scheller B, Speck U, Abramjuk C, Bernhardt U, Bohm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 110(7), 810–814 (2004).
- Albrecht T, Speck U, Baier C, Wolf KJ, Bohm M, Scheller B. Reduction of stenosis due to intimal hyperplasia after stent supported angioplasty of peripheral arteries by local administration of paclitaxel in swine. Invest. Radiol. 42(8), 579–585 (2007).
- Cremers B, Speck U, Kaufels N et al. Drug-eluting balloon: very short-term exposure and overlapping. Thromb. Haemost. 101(1), 201–206 (2009).
- Scheller B, Hehrlein C, Bocksch W et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 355(20), 2113–2124 (2006).
- Scheller B, Hehrlein C, Bocksch W et al. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxelcoated balloon catheter. Clin. Res. Cardiol. 97(10), 773–781 (2008).
- Schnorr B, Speck U, Scheller B. Review of clinical data with Paccocath®-coated balloon catheters. Minerva Cardioangiol. 59(5), 431–445 (2011).
- Tepe G, Zeller T, Albrecht T et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N. Engl. J. Med. 358(7), 689–699 (2008).
- Werk M, Langner S, Reinkensmeier B et al. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 118(13), 1358–1365 (2008).
- Freyhardt P, Zeller T, Kroncke TJ et al. Plasma levels following application of paclitaxel-coated balloon catheters in patients with stenotic or occluded femoropopliteal arteries. Rofo 183(5), 448–455 (2011).
- Schmidt A, Ulrich M, Winkler B et al. Angiographic patency and clinical outcome after balloon-angioplasty for extensive infrapopliteal arterial disease. Catheter Cardiovasc. Interv. 76(7), 1047–1054 (2011).
- Diehm N, Sin S, Hoppe H, Baumgartner I, Buchler P. Computational biomechanics to simulate the femoropopliteal intersection during knee flexion: a preliminary study. J. Endovasc. Ther. 18(3), 388–396 (2011).
- Scheinert D, Scheinert S, Sax J et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J. Am. Coll. Cardiol. 45(2), 312–315 (2005).
- Gray WA, Granada JF. Drug-coated balloons for the prevention of vascular restenosis. Circulation 121(24), 2672–2680 (2010).
▪ Interdisiciplinary peripheral arterial disease treatment guidelines. Will be revised soon.
▪▪ Excellent review article on contemporary endovascular therapy of the superficial femoral artery.
▪ Landmark experimental article on drug-eluting balloon angioplasty.
▪ Landmark experimental article on drug-eluting balloon angioplasty.
▪ Pioneering randomized clinical study on paclitaxel-coated balloons in the femoropopliteal arteries.
▪ Landmark randomized clinical study on paclitaxel-coated balloons in the femoropopliteal arteries.
▪ Website
101 Millenium Research Group. US Markets for Peripheral Vascular Devices 2011. http://mrg.net