Research Article - Journal of Interventional Nephrology (2023) Volume 6, Issue 4
Correlation Between Arteriovenous Fistula Flow Patterns and Impaired Cardiac Systolic Function in Hemodialysis Patients with End Stage Kidney Disease.
Riyadh M Al-Saegh1*, Mohammed A Al-Kubaisy2, Maha A Al-Mukhtar3
1Professor of Nephrology and Kidney Transplantation, Department of Medicine, College of Medicine, University of Kerbala, MOHE, Iraq
2Internal Medicine specialist, Department of Internal Medicine, Al-Hassan Al-Mujtaba Hospital, MOH, Iraq
3Radiologist, Department of Radiology, Alkafeel Super-specialty hospital, Karbala, Iraq
Professor of Nephrology and Kidney Transplantation, Department of Medicine, College of Medicine, University of Kerbala, MOHE, Iraq
E-mail: Riyadh.alsaegh@uokerbala.edu.iq
Received: 21-July-2023, Manuscript No.oain-23- 110905; Editor assigned: 24-July-2023, Pre-QC No.oain-23- 110905 (PQ); Reviewed: 7-August-2023, QC No.oain-23- 110905; Revised: 14-August-2023, Manuscript No.oain-23- 110905 (R); Published: 23-August-2023; DOI: 10.47532/oain.2023.6(4).129-135
Abstract
Abstract Background Cardiovascular disease stands as the leading cause of mortality and morbidity among patients with chronic kidney disease. Notably, arteriovenous fistulas show the highest long-term patency rate when compared to alternative hemodialysis access methods. However, the creation of these fistulas raises concerns about potential adverse effects on cardiac structure and function. These physiological consequences could escalate to the point of myocardial decompensation, leading to decreased ejection fraction and potentially resulting in left ventricular hypertrophy and/or heart failure. Method Conducted at Imam Al-Hussein Medical City and Imam Al-Hassan Hospital in the city of Karbala, this cross-sectional study spanned from January to June 2022. The study enrolled a cohort of one hundred fifty patients diagnosed with end stage kidney disease on regular hemodialysis schedules. Each participant underwent color Doppler ultrasonography to assess fistula characteristics, alongside transthoracic echocardiography for a comprehensive evaluation of cardiac function. Results The study findings revealed that the prevalence of high flow fistulas among the participants was 41.3% (62 patients), while 50% exhibited normal flow (75 patients), and 8.7% (13 patients) demonstrated low flow characteristics. Conclusion Notably, a statistically significant association emerged between the type of flow observed in arteriovenous fistulas and the systolic function of the heart (P-value = 0.032). Patients with high flow velocity fistulas displayed a notably higher prevalence of reduced systolic function. Moreover, patients with fistulas situated in the arm exhibited greater flow velocities when compared to those with forearm fistulas.
Keywords
Arteriovenous fistula • High flow • Systolic dysfunction • Hemodialysis
Introduction
Arteriovenous fistula (AVF) placement for hemodialysis (HD) can lead to a cascade of physiological changes that impact cardiac function, potentially leading to high-output heart failure (HOHF) [1]. The interplay between systemic vascular resistance, myocardial contractility, preload, stroke volume, and heart rate results in an initial surge in cardiac output immediately following AVF creation [2,3]. The subsequent arterialization process, driven by increased pressure transmission from the artery to the vein through the fistula, triggers vascular wall thickening over a period of 4-6 weeks [4]. This vascular remodeling is accompanied by a concomitant rise in blood volume and the release of atrial and brain natriuretic peptides [5,6]. As the AVF matures and blood flow escalates, alterations in atrial and ventricular dimensions and function become evident, contributing to heightened left ventricular filling pressures [5-8]. These physiological adaptations can potentially progress to myocardial decompensation, left ventricular dilatation, reduced ejection fraction, and the development of left ventricular hypertrophy (LVH) or heart failure (HF) [5-9]. Notably, these changes superimpose upon the existing LVH, dilatation, and dysfunction often seen in uremic cardiomyopathy [8].In the realm of vascular access, the definition of a high flow access remains elusive. The National Kidney Foundation Kidney Dialysis Outcome Quality Initiative (NKF/KDOQI) and European Best Practice Guidelines do not delineate reference ranges for HFA. In contrast, the Vascular Access Society (VAS) characterizes HFA as having a flow rate between 1 and 1.5 L/min or exceeding 20% of cardiac output [7]. Notably, AVFs with flows surpassing 2 L/min, particularly those located in the upper arm, are more prone to precipitating HF [7-11]. The emergence of HF symptoms serves as a pivotal indicator of excessive AVF flow. Nevertheless, it is noteworthy that high-flow AVF may manifest even without overt HF symptoms, with flows exceeding 1500–2000 ml/min, as posited by select experts [12,13]. Prolonged periods following AVF creation, spanning months or even years, can witness a surge in flow velocity attributed to ongoing vascular remodeling, particularly within the confines of the feeding artery and arteriovenous anastomosis [14]. This study looks to shed light on this critical issue and provide insights into the potential implications for patient care. The interplay between AVF flow dynamics and their impact on cardiac function underscores the importance of investigating the prevalence of systolic dysfunction in patients undergoing HD with high-flow AVFs. The recognition of such subtle alterations holds paramount significance for optimizing patient outcomes and refining clinical interventions in the management of HD patients with AVFs.
Study design and setting
A cross-sectional study was meticulously conducted at Imam Al-Hussein Medical City and Imam Al-Hassan Hospital, situated in the city of Karbala, commencing from January to June 2022. This duration was chosen to provide a comprehensive perspective on the subject matter.
Sample selection
The study enrolled a well-defined study of 150 Iraqi patients, each exhibiting AVF as their designated vascular access for HD. Ensuring the integrity of the sample, only patients whose AVF patency was confirmed through the palpation of thrill were included. A judicious approach was undertaken to exclude individuals with a history of ischemic heart disease, valvular heart disease, pre-existing HF, or any symptoms indicative of cardiac anomalies prior to AVF creation. Furthermore, patients who experienced clinical AVF failure and those with incomplete data were thoughtfully excluded from the study, aligning with the rigorous standards of credible research.
Data Collection and Instrumentation
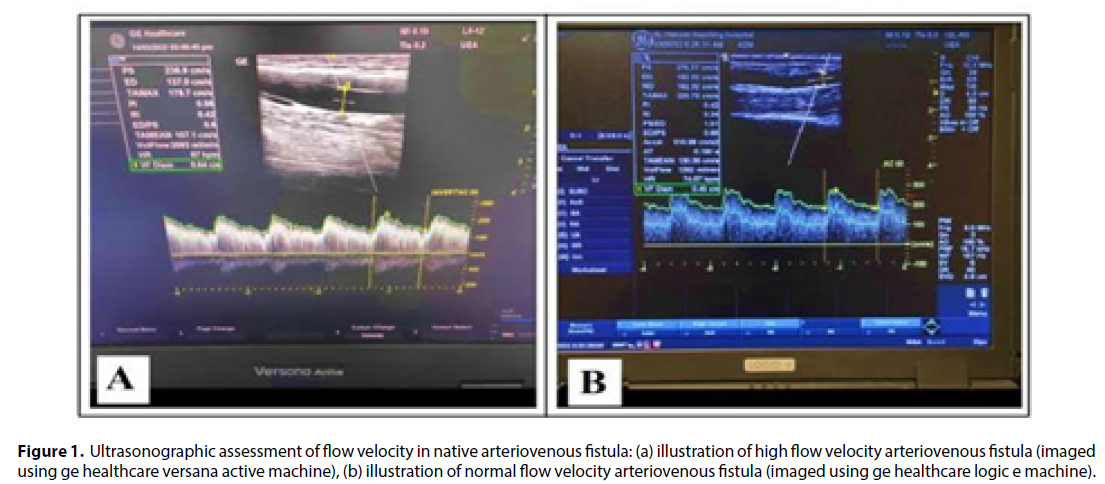
A meticulously prepared preconstructed data collection sheet served as the foundation for accurate and comprehensive data capture. The state-of-the-art General Electric Healthcare Doppler ultrasound machines, equipped with a linear array probe and frequencies ranging from 6-12 MHz, were employed for meticulous assessment of AVF flow. Importantly, the Doppler angle was meticulously kept at ≤ 60°, thus ensuring precise measurements [15]. To eliminate any potential oversight of low-speed blood flow, the entire vascular diameter was thoughtfully measured along the vascular wall [16,17], as illustrated in (Figure 1).
Figure 1: Ultrasonographic assessment of flow velocity in native arteriovenous fistula: (a) illustration of high flow velocity arteriovenous fistula (imaged using ge healthcare versana active machine), (b) illustration of normal flow velocity arteriovenous fistula (imaged using ge healthcare logic e machine).
Flow Velocity Measurement
The flow velocity measurements were derived from a mathematical formula [15-16].
Qa (ml/min) =TAMEAN (cm/s) x section area * π r^2 (cm^2) x 60.
This comprehensive approach harnessed key parameters including flow velocity (Qa), timeaveraged mean velocity (TAMEAN), 3.14 (π), and the inner diameter (r).
Echocardiographic Assessment
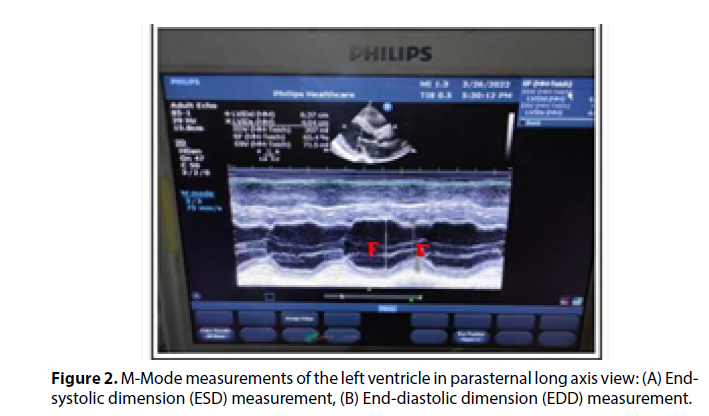
The assessment of echocardiographic parameters was executed with the utmost precision and sophistication. Doppler echocardiography machines (Philips Healthcare) in conjunction with a S5-1-MHz transducer were employed for this purpose. The dimensions of the left ventricle were meticulously measured using 2D-guided M-mode images, focusing on pivotal parameters such as end-diastole and endsystole (Figure 2).
Ejection Fraction Calculation
The determination of EF served as a cornerstone of this study’s methodology, capturing vital cardiac dynamics. Employing a meticulous formula:
EF = (EDV - ESV / EDV) * 100%, wherein EDV stands for end-diastolic volume and ESV signifies end-systolic volume [17].
Data Analysis
The resultant data were analyzed using the IBM SPSS software for Microsoft Windows, version 26. The chi-square test and ANOVA test, wellestablished statistical methodologies, were used. Notably, the threshold for statistical significance was set at P ≤ 0.05.
Definitions
Classification of Left Ventricular Systolic Function (As Per American College of Cardiology - ACC)
• Hyperdynamic: Left Ventricular Ejection Fraction (LVEF) < 70%
• Normal: LVEF 50% - 70%
• Mild Dysfunction: LVEF 40% - 49%
• Moderate Dysfunction: LVEF 30% - 39%
• Severe Dysfunction: LVEF ≤ 30% (20)
Classification of AVF Flow Velocity (Following NKF/KDOQI Criteria)
• Low Flow: > 600 ml/min
• Normal Flow: 600 ml/min to 1500 ml/min
• High Flow AVF: ≤ 1500 ml/min [7-18].
Results and Discussion
This study encompassed one hundred fifty patients diagnosed with chronic kidney disease undergoing HD, utilizing AVF as their vascular access. Among these individuals, eighty-seven patients (58%) were males, while 63 (42%) were females. The mean age of the participants was 50.39 ± 13.9 years. Our findings indicated that the prevalence of high flow velocity AVF was 41.3%, using a cutoff point of 1500 ml/min, with a mean velocity flow of 2269.4 ± 683.7 for high flow AVF, 963.3 ± 275.9 for normal flow AVF, and 460.7 ± 101.1 for low flow AVF Table 1 Saleh et al. [19] in a study from 2018, reported a prevalence of 24% for high flow AVF, using a cutoff point of 2000 ml/min, with a mean flow of 3430.13 ± 1256.28 ml/min for the high flow AVF group compared to 958.63 ± 487.35 ml/ min for the non-high flow AVF group. Schier et al. [20] conducted a study in 2013, evaluating AVF closure due to HOHF in renal transplant recipients. Among 113 patients, 25.7% needed AVF closure due to HF symptoms. The AVF closure group showed a mean Qa (flow rate) of 2197.2 ml/min, contrasting with 850.9 ml/ min in the non-closure group. Specific AVF or patient characteristics might predispose individuals to HF.
AVF flow velocity |
No. | % | Mean ± SD (ml/min.) |
|---|---|---|---|
| Low | 13 | 8.7 | 460.7 ± 101.1 |
| Normal | 75 | 50 | 963.3 ± 275.9 |
| High | 62 | 41.3 | 2269.4 ± 683.7 |
| Total | 150 | 100 | 1459.2 ± 436.6 |
| AVF= Arteriovenous fistula, SD: Standard deviation | |||
Table 1. Distribution according to AVF flow velocity.
The current study shows a statistically significant adverse impact of chronic diseases (diabetes mellitus and hypertension), and AVF site on the flow velocity (P-value < 0.05 using Chi-square test). Conversely, no significant association was found between gender, family history of CKD, number of HD sessions per week, number of AVF created, and flow velocity (P-value > 0.05 using Chi-square test) (Table 2). Our study found no statistically significant relationship between body mass index and Qa (P-value > 0.05). Notably, the relationship between body mass index and Qa observed in our study differs from a similar study in Egypt, which found a significant association between these two variables [19]. In our study, both arm and forearm AVF patients were included. We measured AVF Qa using the brachial artery for arm AVFs, consistent with Lomonte et al.’s approach [21], which shows a proven correlation with AVF flow velocity. However, for forearm AVFs, measuring Qa through the radial artery might lead to underestimations due to the contribution of ulnar artery blood flow through the palmar arch. Studies suggest arm AVFs have higher Qa, potentially up to twice that of forearm fistulas [7-22]. In our study, 46.3% of patients with arm AVFs exhibited Qa values surpassing 1500 ml/min, while this percentage was 18.5% for those with forearm AVFs and high Qa. Similar trends were seen in Basile et al.’s 2016 cross-sectional study involving 56 lower arm AVF patients and 30 upper arm AVF patients. The upper arm AVFs were associated with a statistically significant increase in Qa and a decrease in systemic vascular resistance compared to lower arm AVFs [23-25].
Variable |
Flow velocity | Total | P. value* |
|||
|---|---|---|---|---|---|---|
| Low | Normal | High | ||||
| Gender |
Male | 9 | 38 | 40 | 87 | 0.182 |
| Female | 4 | 37 | 22 | 63 | ||
| Body mass index |
Normal | 10 | 39 | 27 | 76 | 0.225 |
| Overweight | 1 | 24 | 24 | 49 | ||
| Obese | 2 | 12 | 11 | 25 | ||
| Chronic diseases |
Negative | 1 | 7 | 6 | 14 | <0.001 |
| DM | 0 | 2 | 0 | 2 | ||
| HTN | 5 | 31 | 50 | 86 | ||
| DM + HTN | 7 | 35 | 6 | 48 | ||
| Family history of CKD |
Positive | 1 | 11 | 11 | 23 | 0.642 |
| Negative | 12 | 64 | 51 | 127 | ||
| Number of hemodialysis sessions per week |
1 | 0 | 5 | 2 | 7 | 0.739 |
| 2 | 6 | 34 | 32 | 72 | ||
| 3 | 7 | 36 | 27 | 70 | ||
| 4 | 0 | 0 | 1 | 1 | ||
| AVF site |
Arm | 6 | 60 | 57 | 123 | <0.001 |
| Forearm | 7 | 15 | 5 | 27 | ||
| AVF number |
1 | 12 | 63 | 57 | 132 | 0.497 |
| 2 | 1 | 10 | 4 | 15 | ||
| 3 | 0 | 2 | 0 | 2 | ||
| 4 | 0 | 0 | 1 | 1 | ||
| Total | 13 | 75 | 62 | 150 | ||
| *Chi-square test used in comparison. DM: diabetes mellitus, HTN: hypertension, CKD: chronic kidney disease. | ||||||
Table 2. Association between variables and AVF flow velocity.
Moreover, no statistically significant relationships were seen between patient age, duration of CKD, duration of AVF since creation, and flow of AVF (Table 3). Another investigation conducted in 2018 included 100 chronic end-stage renal disease patients undergoing HD at Ain Shams University’s dialysis center in Egypt 19. The Egyptian study encompassed 34 females and 66 males, with an average age of 48.48 ± 13.75 years. Its analysis proved that both age and gender did not show statistically significant relationships with flow velocity in AVFs, aligning with our study’s findings. Our study unveiled that patient with high flow velocity AVF displayed lower EF compared to a joint group of normal and low flow AVF patients, with a mean value of 60 ± 8.9% versus 63 ± 8.3% Table 4 Saleh et al. 19 similarly reported a reduced EF in the high flow AVF group (mean value of 57.32%) compared to the non-high flow AVF group (mean value of 62.90%), establishing a statistically significant correlation between high flow AVF and compromised left ventricular function. Furthermore, 12% (8 patients) in our study showed LV systolic dysfunction (EF < 55%). These findings collectively emphasize the intricate relationship between AVF flow patterns and cardiac function, reinforcing the importance of careful assessment and management in patients with end stage kidney disease. In a separate study conducted in China in 2013, fifty HD patients utilizing AVF as their vascular access were enrolled. Cardiac output, cardiac index, and peripheral vascular resistance were assessed using the ultrasound dilution technique. The findings proved a significant increase in both cardiac output and cardiac index, along with a reduction in peripheral vascular resistance, when the AVF Qa exceeded 2 l/min. The study’s echocardiographic analysis showed that as Qa increased, there was a corresponding reduction in EF [24,25]. Building upon these insights, the present study relies on EF estimation through M-mode echocardiography as a means of assessing cardiac systolic function in HD patients with AVF. This approach offers ease of measurement while yielding results that are consistent with earlier observations.
| Variable | Flow velocity | No. | Mean ± SD | P. value |
|---|---|---|---|---|
| Age of patients (years) |
Low | 13 | 50.54 ± 13.10 | 0.134 |
| Normal | 75 | 52.55 ± 13.60 | ||
| High | 62 | 47.74 ± 14.20 | ||
| Total | 150 | 50.39 ± 13.90 | ||
| Duration of CKD (years) |
Low | 13 | 2.65 ± 1.90 | 0.574 |
| Normal | 75 | 3.82 ± 4.20 | ||
| High | 62 | 3.67 ± 3.10 | ||
| Total | 150 | 3.66 ± 3.60 | ||
| AVF duration (years) |
Low | 13 | 1.76 ± 1.60 | 0.13 |
| Normal | 75 | 1.89 ± 1.50 | ||
| High | 62 | 2.52 ± 2.30 | ||
| Total | 150 | 2.14 ± 1.90 | ||
| ANOVA test used in all comparisons. AVF: arteriovenous fistula, SD: Standard deviation. | ||||
Table 3. Association between variables and AVF flow velocity.
Systolic function of the heart |
AVF flow velocity | Total | P. value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Normal | High | |||||||||
| No. | % | No. | % | No. | % | No. | % | ||||
| Mild dysfunction | 3 | 2 | 2 | 1.3 | 8 | 5.3 | 13 | 8.6 | 0.032 | ||
| Normal | 7 | 4.7 | 60 | 40 | 48 | 32 | 115 | 76.7 | |||
| Hyper- dynamic | 3 | 2 | 13 | 8.7 | 6 | 4 | 22 | 14.7 | |||
| Total | 13 | 8.7 | 75 | 50 | 62 | 41.3 | 150 | 100 | |||
| EF (Mean ± SD) | 63 % ± 8.3 % | 60 % ± 8.9 % | 61.7 % ± 8.1 | ||||||||
| P. value estimated by using Chi-square test, SD: Standard deviation | |||||||||||
Table 4. Association between AVF flow velocity and systolic function of the heart.
Conclusion
The investigation revealed a notable prevalence of high flow velocity AVFs, with 41.3% of participants exceeding the 1500 ml/min threshold. The observed correlation between high flow velocity AVFs and compromised cardiac systolic function underscores the potential implications for cardiovascular health in this patient population. Comparisons between arm and forearm AVFs highlighted the significance of measurement techniques, with Qa assessments through the brachial artery yielding more correct results in arm AVFs. Notably, the study’s findings were consistent with previous research in terms of age and gender not being significant factors affecting flow velocity.
Recommendations
The complex relationship between chronic diseases, such as hypertension and diabetes mellitus, and Qa values emphasized the need for comprehensive patient evaluation.
Ethical approval
Data collection and patient’s enrollment were following Declaration of Helsinki of World Medical Association, 2013 for the ethical principles of research involving human. Signed informed consent was obtained from each participant and data were kept confidentially.
References
- Bargman JM, Skorecki KL Chapter 305: chronic kidney disease. In: Kasper DL, Fauci AS, Hauser S., Longo DL, Jameson JL, & Loscalzo J Harrison’s Principles of Internal Medicine Volume 1. 20th Ed. New York Chicago San Francisco McGraw Hill Education; 2116-2123 (2018).
- Guyton AC, Sagawa K Compensations of cardiac output and other circulatory functions in areflex dogs with large A-V fistulas. Am J Physiol. 1961, 1157-1163.
- Savage MTessa, Ferro CJ, Sassano A et al. Tomson CRV. The impact of arteriovenous fistula formation on central hemodynamic pressures in chronic renal failure patients: A prospective study. American Journal of Kidney Diseases. 2002, 753-759.
- Conway B, Phelan PJ, Stewart GD et al. Chapter 15: Nephrology and urology. In: Ralston SH, Penman ID, Strachan MWJ, Hobson RP. Davidson’s Principles and Practice of Medicine. 23rd ed. Churchill Livingstone/Elsevier. 422-423 (2018).
- Iwashima Y, Horio T, Takami Y et al. Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis. 40, 974-982 (2002).
- Ori Y, Korzets A, Katz M et al. Hemodialysis arteriovenous access—a prospective hemodynamic evaluation. Nephrology Dialysis Transplantation. 11, 94-97 (1996).
- Basile C, Lomonte C, Vernaglione L et al. The relationship between the flow of arteriovenous fistula and cardiac output in hemodialysis patients. Nephrol Dial Transplant. 23,282-287 (2008).
- Parfrey PS, Foley RN, Harnett JD et al. Outcome and risk factors for left ventricular disorders in chronic uremia. Nephrol Dial Transplant. 11, 1277-1285 (1996).
- Ori Y, Korzets A, Katz M et al. The contribution of an arteriovenous access for hemodialysis to left ventricular hypertrophy. Am J Kidney Dis. 40, 745-752 (2002).
- Martínez Gallardo R, Ferreira Morong F, García Pino G et al. Congestive heart failure in patients with advanced chronic kidney disease: association with pre-emptive vascular access placement. Nefrologia.32, 206-212 (2012).
- Wijnen E, Keuter XH, Planken NR et al. The relation between vascular access flow and different types of vascular access with systemic hemodynamics in hemodialysis patients. Artif Organs. 29, 960-964 (2005).
- Malik J Heart disease in chronic kidney disease – review of the mechanisms and the role of dialysis access. The Journal of Vascular Access. 19, 3-11 (2018).
- Basile C, Lomonte C. The complex relationship among arteriovenous access, heart, and circulation. Seminars in Dialysis. 31, 15-20 (2017).
- Amerling R, Ronco C, Kuhlman M et al. Arteriovenous Fistula Toxicity. Blood Purification. 31, 113-120(2011).
- Ren C, Chen J, Wang Y et al. Application of ultrasonography in monitoring the complications of autologous arteriovenous fistula in hemodialysis patients. Medicine. 97, e12994 (2018).
- Ibeas J, Vallespin J. Chapter 3: Arteriovenous Fistula Ultrasound Examination. In: Iglesias R, Vallespin J, and Ibeas J. Handbook on Ultrasound for Vascular Access Examination- From the Specialist to the Nurse. EDTNA/ERCA.59-64 (2018).
- Otto CM. Chapter 6: Left and Right Ventricular Systolic Function. In: Otto CM. Textbook of Clinical Echocardiography. 6th ed. Elsevier.148-152 (2018).
- National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis.48,S1-S322 (2006).
- Saleh MA, El Kilany WM, Keddis VW et al. Effect of high flow arteriovenous fistula on cardiac function in hemodialysis patients. Egypt Heart J. 70, 337-341 (2018).
- Schier T, Göbel G, Bösmüller C et al. Incidence of arteriovenous fistula closure due to high-output cardiac failure in kidney-transplanted patients. Clin Transplant. 27, 858865 (2013).
- Lomonte C, Casucci F, Antonelli M et al. Is there a place for duplex screening of the brachial artery in the maturation of arteriovenous fistulas? Semin Dial. 18, 243-246 (2005).
- Begin V, Ethier J, Dumont M et al. Prospective evaluation of the intra-access flow of recently created native arteriovenous fistulae. Am J Kidney Dis. 40, 1277-1282 (2002).
- Basile C, Vernaglione L, Casucci F et al. The impact of hemodialysis arteriovenous fistula on hemodynamic parameters of the cardiovascular system. Clin Kidney J. 9, 729-734 (2016).
- Ye WL, Fang LG, Ma J et al. Long-term effects of arteriovenous fistula on cardiac structure and function in non-diabetic hemodialysis patients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 35, 95-101 (2013).
- Al Kubaisy MA, Al Saegh RM, Makki MM et al. The Prevalence of Systolic Dysfunction in Patients on Hemodialysis with High Flow Arteriovenous Fistula. AJMS. 9, 151-62 (2003).
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar
Indexed at, Crossref, Google Scholar