Case Report - Interventional Cardiology (2016) Volume 8, Issue 5
Coronary artery steal syndrome in patient after coronary arteries by-pass surgery and left subclavian artery stenting-is the maintenance of a patent internal mammary artery still necessary?
- Corresponding Author:
- Rafal Januszek
2nd Department of Cardiology and Cardiovascular Interventions, University Hospital, Krakow, Poland
Tel: +48124247170
E-mail: jaanraf@interia.pl
Submitted: 19 September 2016; Accepted: 05 October 2016; Published online: 10 October 2016
Abstract
The use of the left internal mammary artery (LIMA) is preferred for the revascularization of a stenosed or occluded left anterior descending artery (LAD). Occlusion or stenosis of proximal part of the left subclavian artery (LSA) could induce reverse blood flow in LIMA called coronary subclavian steal syndrome (CSSS) and should be revascularized before coronary artery bypass grafting (CABG) surgery with LIMA. If restenosis of previously stented LSA occurs after CABG surgery with LIMA another revascularization is inevitable. However, the revascularization of LSA could reveal that increased blood pressure in LAD persistent through years due to present CSSS could substantially influence blood pressure change in coronary arteries and LIMA graft. This phenomenon can raise the question if it is necessary to maintain patent LIMA-LAD graft.
Keywords
Coronary artery disease.
The use of the left internal mammary artery (LIMA) is preferred for the revascularization of a stenosed or occluded left anterior descending artery (LAD). This is predicated mainly by the LIMA resistance against atherosclerosis and relatively long patency time in comparison to venous bypass grafts [1]. Occlusion or stenosis of proximal part of the left subclavian artery (LSA) could induce reverse blood flow in LIMA called coronary subclavian steal syndrome (CSSS) and was described for the first time by Harjola and Valle in the 1974 [2]. It is mainly caused by atherosclerosis but could also occur in Takayasu’s arteritis, radiation arteritis, thoracic outlet syndrome, giant cell arteritis or congenital aortic abnormalities. The subclavian artery stenosis frequency in patients qualified for coronary artery bypass grafting surgery (CABG) is estimated on 0.2- 6.8% [3].
Case
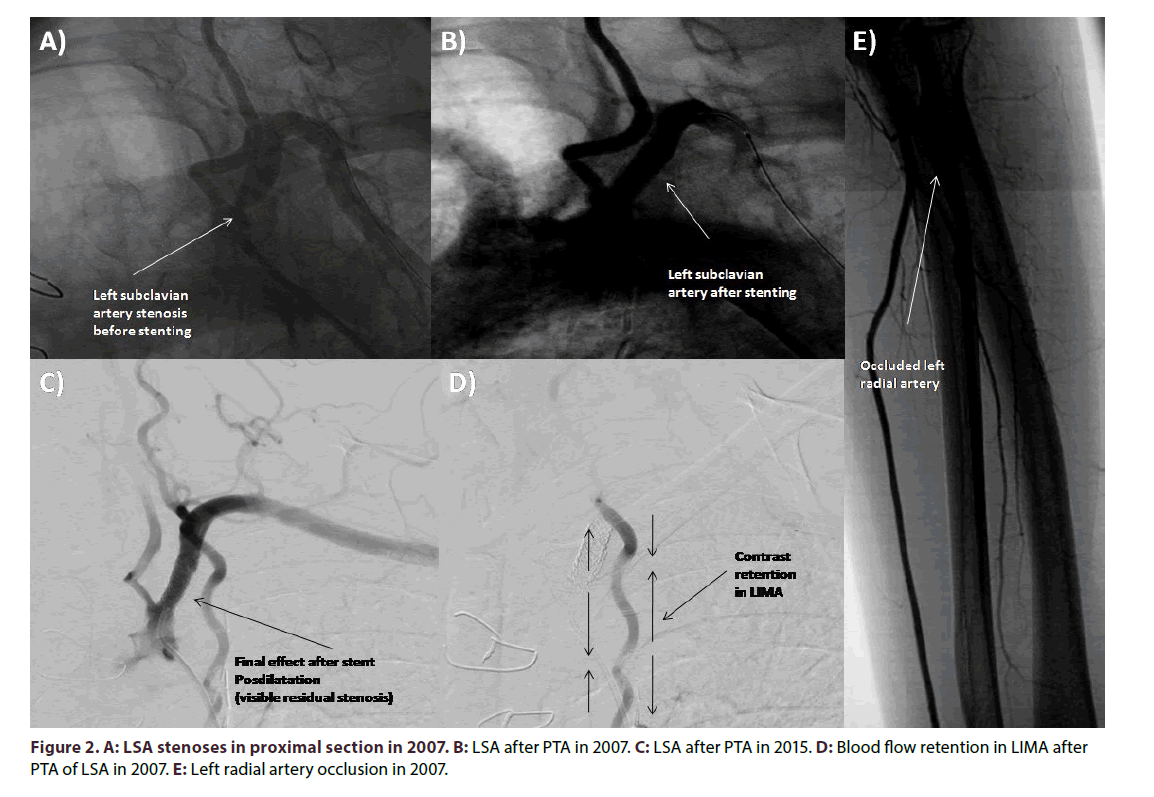
A 63-year-old woman with coronary artery disease (CAD) was admitted due to exacerbation of exertional chest pain and concomitant left upper limb ischemia symptoms including pulslessness, parenthesis, claudication, pallor and coldness. She underwent CABG ten years ago with implantation of arterial graft - LIMA to LAD and venous graft to the marginal (Mg) branch of the circumflex coronary artery (Cx) due to the 50% stenoses all along the left main coronary artery (LMCA) and 60% ostial LAD stenoses, 70% ostial Cx stenoses and 60% stenoses of first Mg (Figure 1A). There were no significant atherosclerotic lesions in the right coronary artery (RCA). The Syntax Score that time was 27. The supra-aortic arteries ultrasonography showed no pathology, except parietal atherosclerosis. Around two years after CABG procedure the patient underwent percutaneous transluminal angioplasty (PTA) of LSA. The PTA was performed with bare metal stent (Express Vascular SD) implantation due to symptoms of left upper limb ischemia caused by significant stenosis in the proximal part of LSA (Figures 2A and 2B). The systolic over the diastolic blood pressure measured before the procedure on the left upper limb was 90/60 mmHg and on the right 145/90 mmHg. The patient did not complain of angina. Total occlusion of the left radial artery was incidentally diagnosed (Figure 2E). After the LSA PTA arterial blood pressure equalized and left upper limb ischemia symptoms revealed. She also suffered from arterial hypertension, type 2 diabetes treated with oral ant diabetics, hypercholesterolemia, and was heavy smoker with almost 20 cigarettes smoked every day. Moreover, patient had diagnosed peripheral arteries occlusive disease with previous PTA of the left femoral superficial artery, and with significant stenosis of the right external iliac artery. Furthermore, she was diagnosed with stroke of the right hemisphere 3 years before CABG operation.
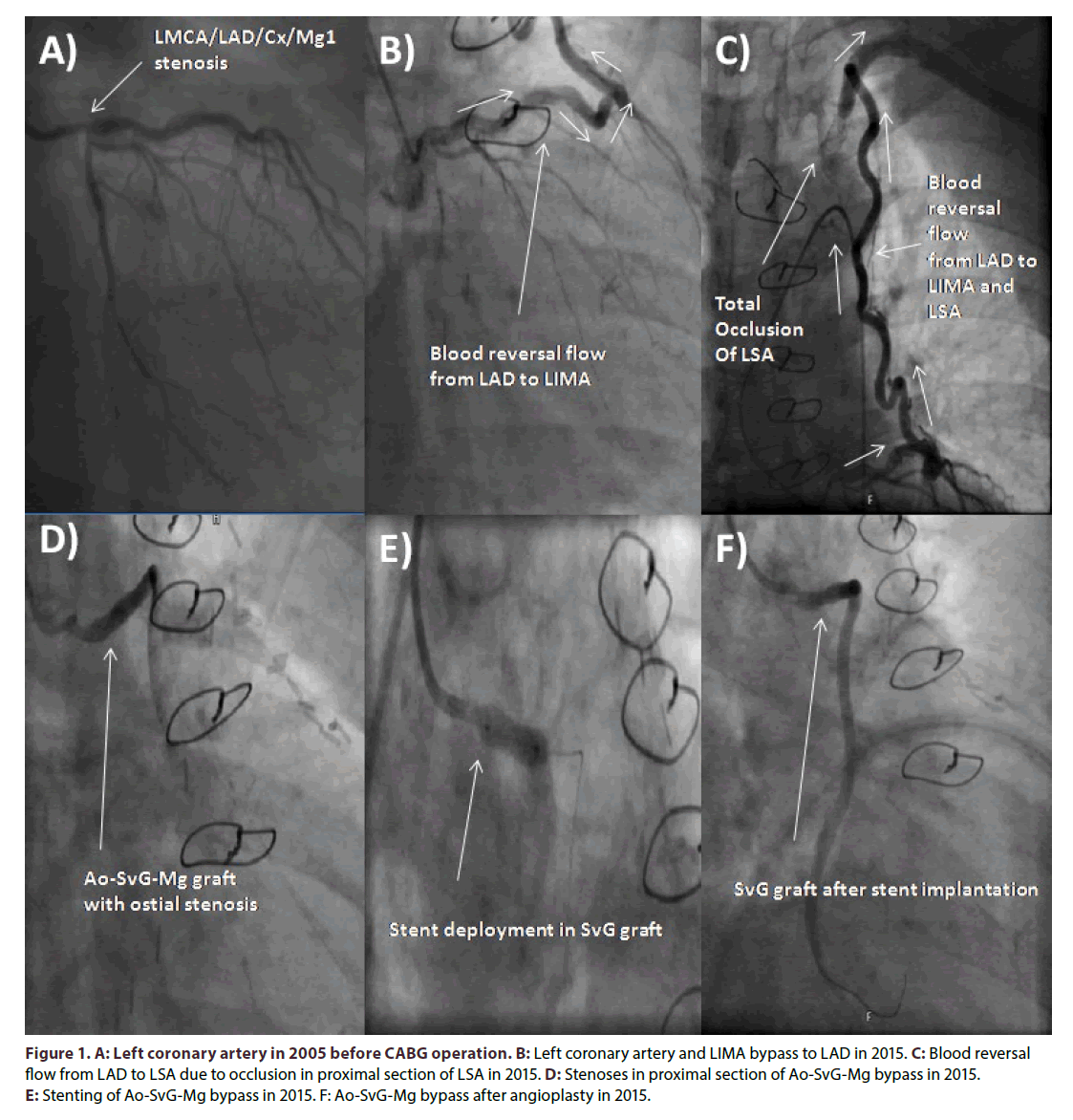
Figure 1. A: Left coronary artery in 2005 before CABG operation. B: Left coronary artery and LIMA bypass to LAD in 2015. C: Blood reversal flow from LAD to LSA due to occlusion in proximal section of LSA in 2015. D: Stenoses in proximal section of Ao-SvG-Mg bypass in 2015. E: Stenting of Ao-SvG-Mg bypass in 2015. F: Ao-SvG-Mg bypass after angioplasty in 2015.
Presently, the echocardiography revealed left ventricle ejection fraction around 60% with slight hypo kinesis of basal segment of inferior wall, mild left atrium enlargement and no significant valvular abnormalities. Angiography of coronary arteries and bypassography showed 40% stenosis of LMCA, up to 50% stenosis in proximal LAD, and reverse blood flow in LIMA-LAD bypass with contrasting of distal part of subclavian artery, occluded Cx in the proximal part, no significant atherosclerosis in RCA, 70% stenosis in ostium of Ao- SvG-Mg by-pass (Figures 1B-1D). Selective contrast injection to LIMA-LAD bypass was not possible due to a total occlusion of previously implanted stent in LSA. The successful percutaneous coronary intervention (PCI) of Ao-SvG-Mg bypass graft was performed with direct implantation of everolimus-eluting stent Xience Pro 4.0 × 12 mm at 16-20 atm (Figures 1E and 1F). Clinical symptoms of exertional stenocardia decreased after PCI, whereas, symptoms of left upper limb ischemia remained. The systolic over diastolic blood pressure on the right side was 145/80 mmHG, whereas on the left 60/0 mmHg. Due to the suspicion of silent LAD-LIMA-LSA steal syndrome revealed by upper limbs exertion the scintigraphy before and after electrocardiographic treadmill test accompanied by upper limbs movements was performed. According to our knowledge there is no standardized protocol for assessing and differentiating ischemia in this particular case. The resting test revealed perfusion impairment of periapical and medial segment of lateral wall and basilar segments of anterior and lateral wall of the left ventricle. Whereas myocardial perfusion scintigraphy with workload of 6.4 METS revealed perfusion impairment of periapical and medial segment of lateral wall of the left ventricle and basal segments of anterior and lateral wall of the left ventricle. The mild intensification of perfusion impairment was visible in anterior and lateral medial segments of the left ventricle. Based on these results and clinical symptoms of heart (slight exertional stenocardia with improvement after PCI of venous graft) and left upper limb ischemia (pulslessness, pins and needle symptoms and exertional numbness, pain, pallor and weakness) we decided to qualify patient for PTA of LSA. Control angiography revealed that left vertebral artery (LVA) branches from the LSA just above the origin of the LSA branch from the aortic arch, and the occlusion is located peripherally to LVA. The successful PTA of LSA from the left femoral access was followed by unsuccessful attempt of patency regaining from the left brachial access. Pre dilatation was performed with balloon catheters Maverick 2.0 × 15 mm at 12- 16 atm., and Sterling 4.0 × 40 mm at 10-12 atm. The Omnilink Elite stent 7.0 × 19 mm was deployed in proximal LSA after bifurcation of left vertebral artery 12-14 atm. Residual restenosis was estimated on 10%. Control coronarography revealed retrograde blood flow into the LIMA-LAD by-pass. According to that fact final post dilatation of stent in LSA was performed 16 atm (Figure 2C). Final angiography revealed almost no flow in LIMA-LAD by-pass (Figure 2D). It could be concluded that permanent blood supply of left upper limb from LAD through LIMA for almost eight years caused dilatation of LMCA and proximal LAD in comparison to those visualized in pre-CABGcoronarography (Figure 1A). Follow-up control visit one month after the PTA has shown almost equal systolic and diastolic blood pressure on both upper limbs (left brachial artery 150/80 mmHg and right brachial artery 140/75 mmHg), and the stent patency was confirmed in ultrasound.
Discussion
Usually, significant subclavian artery stenosis is diagnosed early, before CABG operation [4]. However under diagnosis in preoperative period could be resulted due to selective internal thoracic artery angiography instead of subclavian artery angiography. Additionally, not significant stenosis in angiography (<50%) before CABG could become significant after operation due to the change in blood pressure gradients. The long-term outcomes after PTA for obstructive lesions of proximal subclavian artery in follow-up depend mainly on the type of the lesion (occlusion, stenosis), distribution of target lesion calcification, artery tortuosity, location to local branches, stenting technique and devices used for the procedure [5-7]. The restenosis rate of subclavian artery after PTA ranges from 4.8% to 28% during the mean follow-up period of 20 to 42 months depending on the subgroup of patients [8,9]. The PTA efficacy of subclavian artery stenosis is lower in occluded arteries compared to stenosis [10]. According to the current ESC guidelines on peripheral artery disease only symptomatic lesions should undergo revascularization and the method of choice is PTA [11]. It demands to be reconsidered, whether the significant or borderline and asymptomatic lesions in LSA should be also be considered for angioplasty before CABG operation. Additionally, smoking status and other atherosclerotic risk factors could accelerate atherosclerosis progression in nearest months and years after CABG. Nowadays, there is no consensus about the timing of SAS in patients qualified for CABG and it is suggested that PTA could be performed before, as well as after the CABG [12]. In the current case after second PTA of LSA blood flow through the LIMA by pass almost completely stopped due to the equalization of blood pressure in the SLA and LAD. So than raise the question whether LIMA by-pass patency is still needed? This is not questionable from the point of view of securing the blood supply to the heart muscle, but on the other hand provides a back-up lifeline for the left upper limb perfusion. According to our knowledge there has not been published a case on endovascular occluding of LIMALAD bypass in this special situation so far. There were a few articles published regarding coil embolization of unligated side branches of internal mammary artery or intercostal branches causing steal syndrome and not embolization of internal mammary artery itself [13]. Another option to avoid this course of events in terms of CSSS development is implantation of the saphenous vein by-pass to LAD instead of LIMA-LAD by-pass in patients with subclavian artery stenosis in proximal section or after PTA of proximal section of subclavian artery in the past.
Conclusion
In conclusion, subclavian artery stenosis in patients before and after CABG operations with LIMA grafting remains an important issue and its course vary widely. Long lasting coronary artery steal syndrome could irreversibly influence on primary pressure distribution in coronary and peripheral arteries impacting substantially on the sense of the maintenance of previously artificially implanted by-passes. It is necessary to emphasize, that this represents a single case of this experience and the results of this study could not be extended to a larger sample of patients but in some cases may suggest the modification of the planned treatment.
Ethical Approval
The identity of the patient was absolutely preserved.
Executive summary
• Occlusion or stenosis of proximal part of the left subclavian artery (LSA) could induce reverse blood flow in LIMA called coronary subclavian steal syndrome (CSSS) and should be revascularized before coronary artery bypass grafting (CABG) surgery with LIMA.
• If restenosis of previously stented LSA occurs after CABG surgery with LIMA another revascularization is inevitable.
References
- Tyras DH, Barner HB, Kaiser GC, Codd JE, Pennington DG, Willman VL. Bypass grafts to the left anterior descending coronary artery: saphenous vein versus internal mammary artery. J. Thorac. Cardiovasc. Surg. 80 (3), 327-333 (1980).
- Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 66(4), 436-438 (1974).
- Prasad A, Prasad A, Varghese I, Roesle M, Banerjee S, Brilakis ES. Prevalence and treatment of proximal left subclavian artery stenosis in patients referred for coronary artery bypass surgery. Int. J. Cardiol. 133(1), 109-111 (2009).
- Saleem D, Majdi H. Echocardiographic diagnosis of left subclavian artery stenosis before coronary artery bypass grafting (CABG). Int. J. Cardiol. 203, 30-31 (2016).
- Wada T, Takayama K, Taoka T, et al. Long-term treatment outcomes after intravascular ultrasound evaluation and stent placement for atherosclerotic subclavian artery obstructive lesions. Neuroradiol. J. 27(2), 213-221 (2014).
- Latacz P, Simka M, Kazibudzki M, Ludyga T, Janas P, Mrowiecki T. Optimal interventional treatment in a patient with occlusion of the brachiocephalic trunk and left subclavian artery with "double" steal syndrome. Postep. Kardiol. Inter. 11(4), 341-343 (2015).
- Ertem AG, Kilic H, Arslantas U, Yeter E. Immediate improvement of left internal thoracic artery graft flow after subclavian artery stenting. Postep. Kardiol. Inter. 10(3), 209-210 (2014).
- Sixt S, Rastan A, Schwarzwälder U, et al. Results after balloon angioplasty or stenting of atherosclerotic subclavian artery obstruction. Catheter. Cardiovasc. Interv. 73(3), 395-403 (2009).
- Patel SN, White CJ, Collins TJ, et al. Catheter-based treatment of the subclavian and innominate arteries. Catheter. Cardiovasc. Interv. 71(7), 963-968 (2008).
- Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter. Cardiovasc. Interv. 61(1), 5-11 (2004).
- European Stroke Organisation, Tendera M, Aboyans V, et al. ESC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur. Heart. J. 32(22), 2851-2906 (2011).
- Rogers JH, Calhoun RF 2nd. Diagnosis and management of subclavian artery stenosis prior to coronary artery bypass grafting in the current era. J. Card. Surg. 22(1), 20-25 (2007).
- Chavan A, Mügge A, Hohmann C, Amende I, Wahlers T, Galanski M. Recurrent angina pectoris in patients with internal mammary artery to coronary artery bypass: treatment with coil embolization of unligated side branches. Radiology. 200(2), 433-436 (1996).