Case Series - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 6
Concomitant immunosuppressant use with pegloticase in patients with tophaceous gout - A case series
- Corresponding Author:
- Michael Y. Bessen
Las Vegas School of Medicine
University of Nevada
Las Vegas, Nevada, USA
E-mail: bessen@unlv.nevada.edu
Abstract
Introduction: Treatment of tophaceous gout with pegloticase is often complicated by the production of anti-pegloticase antibodies. These antibodies result in increased risk of infusion reactions, loss of drug efficacy, and high discontinuation rate. Steroids are used in premedication to reduce potential infusion reactions; however prolonged usage of steroids is associated with significant adverse effects. Objective: To describe the use of immunosuppressants to reduce the incidence of infusion reactions with pegloticase treatment and the potential reduction of steroid use in premedication. Methods: We report seven cases of immunosuppressant use, initiated on the day of the first pegloticase infusion, as an adjunct to treatment with pegloticase for tophaceous gout. Results: Patient 1 was treated for tophaceous gout with oral methotrexate initiated at his first infusion. Low serum urate levels were achieved but urate increased on two occasions after two separate lapses in methotrexate compliance. In each instance, initiation of a higher dose of methotrexate restored pegloticase response. The patient tolerated all treatments without infusion reaction. Patients 2-6 were treated for tophaceous gout with oral methotrexate initiated at their first infusions. For Patient 2, methotrexate was switched to azathioprine at her fifth infusion due to fatigue. All five patients achieved low serum uric acid (sUA) levels, completed their treatments, and did not experience infusion reactions. Patient 7 was treated for tophaceous gout with oral cyclosporine initiated at his first infusion. Methotrexate was not used due to elevated liver function enzymes at baseline. He achieved a low sUA level and was able to complete his treatment without experiencing an infusion reaction. All of the patients were able to decrease their steroid use in pre-medication. Six of the seven were able to discontinue steroid use in pre-medication without developing infusion reactions. Conclusion: Concomitant use of immunosuppressive therapy with pegloticase may play a role in preventing infusion reactions, allow reduction of steroids in pre-medication, and result in improved outcomes. We have demonstrated that immunosuppressive therapy may be initiated on the day of the first infusion. Additionally, therapy does not seem to be confined to a single immunosuppressant; thus, immunosuppressive therapy may be tailored to a patient’s needs. The use of concomitant immunosuppressants with pegloticase treatment warrants further study.
Introduction
Gout is a chronic and debilitating condition caused by deposition of monosodium urate crystals and is the most common cause of inflammatory arthritis worldwide [1]. Gout has a high incidence of comorbidities, including well-established associations with cardiovascular and renal disease, as well as all components of metabolic syndrome, which impact patient prognoses and complicate gout treatment [1].
First-line therapies such as allopurinol, febuxostat, and probenecid are used to lower sUA levels but may prove to be insufficient in patients with resistant disease [2]. Urate-lowering regimens aim to prevent gout recurrence in persons with a history of chronic gout, tophi, or nephrolithiasis, and often aim to lower serum urate levels to less than 6 mg/dl [3]. However, many patients are unable to achieve these levels with first-line therapies. Juraschek et al. found that half of all Americans with gout receiving urate lowering therapies have a serum uric acid level above the 6 mg/dl target [4].
Refractory gout can be treated with pegloticase, a recombinant mammalian uricase that catalyzes the oxidation of uric acid into allantoin, which can effectively lower uric acid levels, aid tophus resolution, and reduce the frequency of gout flares [5]. However, antibodies are often produced against the polyethyleneglycol (PEG) moiety of pegloticase or to the uricase itself, resulting in relatively high discontinuation rates. High titer anti-pegloticase antibodies are associated with failure to maintain low serum urate levels and with infusion reactions [5]. Patients may possess pre-existing anti-PEG antibodies due to previous exposure [6]. Baraf et al. have shown that patients receiving pegloticase every 2 and 4 weeks experience a 26% and 42% infusion reaction rate respectively [7]. To date, a number of case reports have demonstrated that the immune response can be attenuated to allow for successful completion of pegloticase treatment [6,8-10].
Case series
We report seven cases of successful completion of pegloticase treatment with use of concomitant immunosuppressants initiated on the day of first infusion.
Patient 1
Patient 1 is a 36-year-old Caucasian male with a 10-year history of gout. He has a history of hypertension. His first 6th months of treatment were described in our previous paper [11]. Due to high uric acid burden, pegloticase treatment was continued for an additional 6 months.
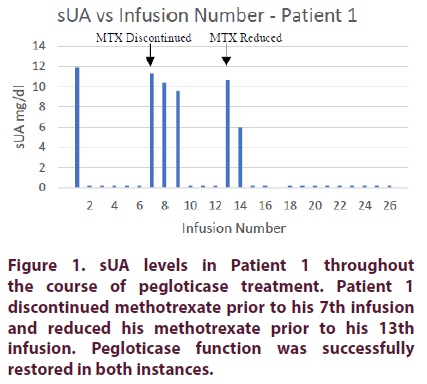
Prior to pegloticase treatment, Patient 1’s sUA was 11.9 mg/dL. On the day of his first pegloticase infusion of 8 mg, the patient was started on oral methotrexate 15 mg per week. He also received premedication consisting of intravenous methylprednisolone 125 mg, IV push diphenhydramine 25 mg, and oral acetaminophen 1000 mg. After the first infusion, the patient achieved an sUA of <0.2 mg/dl. He was able to maintain low serum urate levels for the following five infusions. All six infusions were well tolerated. Prior to the 7th infusion, his sUA increased to 11.3 mg/dl due to missing several doses of oral methotrexate. On the day of his 7th infusion he was given methotrexate 25 mg IM in an attempt to provide a higher level of immunosuppression to compensate for the missed doses. Over the next 6 weeks, the patient continued to receive 25 mg of MTX IM once weekly. His sUA levels gradually declined until an sUA of <0.2 mg/dl was re-achieved prior to his 10th infusion. The patient was then able to complete his first 6 months of treatment while maintaining low sUA levels. He did not experience an infusion reaction during this time period.
At the end of the 6-month treatment course, the patient still had a significant number of large tophi (>5 cm). As such, pegloticase treatment was continued for another 6-month period. Prior to his 13th infusion, the patient decided to go back to taking oral methotrexate 15 mg instead of receiving 25 mg IM weekly. sUA increased to 10.7 mg/dl. Methotrexate 25 mg IM was reinitiated on the day of his 13th infusion. Over the next 4 weeks, sUA levels quickly declined until n sUA of <0.2 was achieved prior to this 15th infusion. The patient maintained this sUA for the remainder of his treatment period (Figure 1).
The patient’s methylprednisolone was decreased to 62.5 mg on his 17th and 18th infusions. It was then discontinued on the 19th infusion; he did not experience any infusion reactions for his remaining 7 infusions.
Patient 2
Patient 2 is a 72-year-old Caucasian female with a 12-year history of gout with tophi on both feet. Her comorbidities include diabetes mellitus type 2, hypertension, and renal insufficiency. Despite treatment with allopurinol, her sUA levels remained high and she continued to have gout attacks.
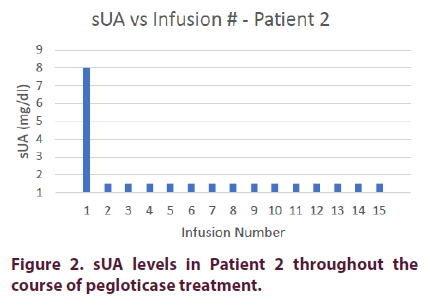
Prior to pegloticase treatment, Patient 2 had an sUA of 8.0 mg/dl. She was started on oral methotrexate 15 mg on the day of her first infusion with premedication consisting of intravenous methylprednisolone 125 mg, IV push diphenhydramine 25 mg, and oral acetaminophen 1000 mg. The patient’s sUA dropped to <1.5 mg/dl after her first infusion. Because of ongoing fatigue, the methotrexate was switched to azathioprine 50 mg BID prior to her 5th infusion.
Additionally, the patient’s methylprednisolone in her premedication was reduced to 62.5 mg on her second infusion because of diabetic exacerbation; her finger-stick glucose level was >400 mg/dl a few hours after receiving her first premedication.
The steroid was further reduced to 31.25 mg on her third infusion and discontinued on her fourth infusion. The patient did not experience any infusion reactions during her remaining 11 infusions (Figure 2).
Patient 3
Patient 3 is a 42-year-old Filipino male with a greater than 10-year history of gout with significant tophus burden on his hands, elbows, knees, and feet. His comorbidities include diabetes mellitus type 2, hypertension, and hyperlipidemia. Despite treatment with allopurinol and febuxostat, he continued to have high levels of sUA and frequent gout attacks.
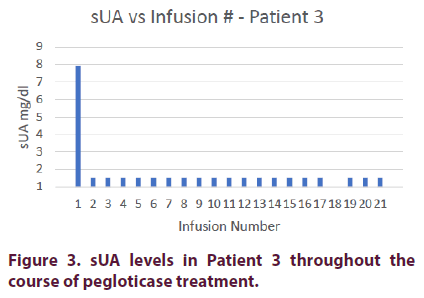
Prior to pegloticase treatment, Patient 3 had an sUA of 7.9 mg/dl. He was started on oral methotrexate 15 mg on the day of his first infusion and was given premedication consisting of intravenous methylprednisolone 125 mg, IV push diphenhydramine 25 mg, and oral acetaminophen 1000 mg. Patient 3 achieved an sUA of <1.5 mg/dl after his first infusion and maintained this level for the remainder of his treatment course.
The patient’s methylprednisolone was decreased to 62.5 mg on his 6th infusion. He was maintained on this dosage until his 16th infusion, when the steroid was discontinued. He did not experience any infusion reactions during his remaining 5 infusions (Figure 3).
Patient 4
Patient 4 is a 48-year-old Caucasian male with a 25-year history of gout. His comorbidities include hypertension and hyperlipidemia. Despite treatment with allopurinol and febuxostat, he continued to have high sUA levels and recurrent gout attacks.
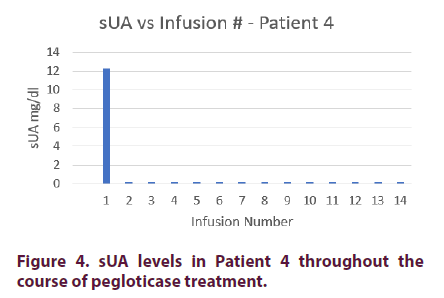
Prior to pegloticase treatment, Patient 4 had an sUA of 12.3 mg/dl. He was started on oral methotrexate on the day of his first infusion and given premedication consisting of intravenous methylprednisolone 125 mg, IV push diphenhydramine 25 mg, and oral acetaminophen 1000 mg. Patient 4 achieved an sUA of <0.2 mg/dl after his first infusion and maintained this level for the remainder of his treatment course.
The patient’s methylprednisolone was decreased to 62.5 mg on his 13th infusion. He never experienced an infusion reaction (Figure 4).
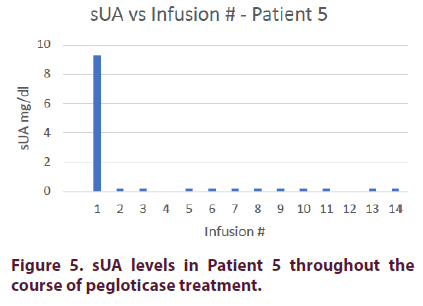
Patient 5
Patient 5 is a 38-year-old Caucasian male with a 13-year history of gout. He also has hyperlipidemia. The patient was unable to tolerate treatment with allopurinol or febuxostat due to development of rash.
Prior to pegloticase treatment, Patient 5 had an sUA of 9.3 mg/dl. He was started on oral methotrexate 15 mg on the day of his first infusion and given premedication consisting of intravenous methylprednisolone 125 mg, IV push diphenhydramine 25 mg, and oral acetaminophen 1000 mg. Patient 5 achieved an sUA of <0.2 mg/dl after his first infusion. Prior to his 9th infusion, his oral methotrexate was reduced to 10 mg due to elevated liver enzymes. He was able to maintain an sUA of <0.2 for his remaining treatment course.
The patient’s methylprednisolone was decreased to 62.5 mg on his 3rd infusion and discontinued on his 7th infusion. He did not experience any infusion reactions during his remaining 7 infusions (Figure 5).
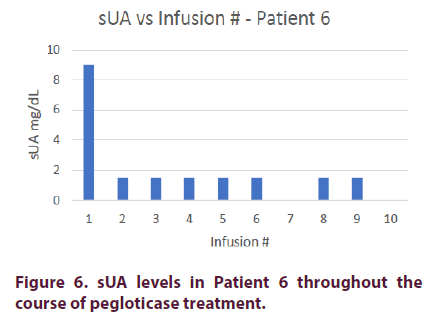
Patient 6
Patient 6 is an 81-year-old Middle Eastern male with an 8-month diagnosed history of gout. His comorbidities include diabetes mellitus, renal insufficiency, and hypertension. He was treated with allopurinol and febuxostat but continued to experience multiple gout flares.
Prior to pegloticase treatment, Patient 6 had an sUA of 9 mg/dl. He was started on oral methotrexate 15 mg on the day of his first infusion and given premedication consisting of intravenous methylprednisolone 125 mg, IV push diphenhydramine 25 mg, and oral acetaminophen 1000 mg. Patient 6 achieved an sUA of <1.5 mg/dl after his first infusion and maintained this level for the remainder of his treatment course. The patient only completed a 4-month course because of a family emergency out of the country. The patient’s methylprednisolone was decreased to 62.5 mg on his 5th infusion and discontinued on his 8th infusion. He did not experience any infusion reactions during his remaining 2 infusions (Figure 6).
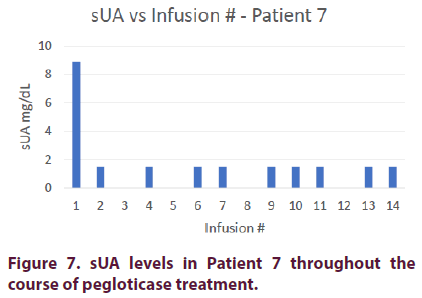
Patient 7
Patient 7 is a 41-year-old Caucasian male with a 4-year history of gout. His comorbidities include hypertension and hyperlipidemia. The patient also had elevated liver enzymes due to nonalcoholic liver disease. He was unable to tolerate treatment with allopurinol or febuxostat due to rising liver enzyme levels.
Prior to pegloticase treatment, Patient 7 had an sUA of 8.9 mg/dl. He was started on oral cyclosporine 50mg BID and given premedication consisting of intravenous methylprednisolone 125 mg, IV push diphenhydramine 25 mg, and oral acetaminophen 1000 mg. Patient 7 achieved an sUA of <1.5 mg/dl after his first infusion and maintained this level for the remainder of his treatment course.
The patient’s methylprednisolone was decreased to 62.5 mg on his 5th infusion and discontinued on his 8th infusion. He did not experience any infusion reactions for his remaining 4 infusions (Figure 7).
Results and discussion
Immunosuppressant use
These seven cases demonstrate improved outcomes of pegloticase treatment for tophaceous gout with concomitant use of immunosuppressants. Patients 1-6 were treated with methotrexate. Patient 2 was switched to azathioprine and Patient 7 was treated with cyclosporine due to elevated liver enzymes. 6 of the 7 patients were able to maintain low serum sUA throughout their treatment courses; Patient 1 experienced two episodes of elevated sUA due to missed or reduced methotrexate dosages. Each of the seven patients experienced reduction of their tophaceous burden and were able to complete their treatment course without experiencing infusion reactions.
Several case reports have demonstrated a possible role for immunosuppressant use with pegloticase to maintain sUA levels, prevent infusion reactions, and avoid discontinuation of pegloticase therapy. In Hershfield et al’s 2014 trial on pegloticase in 30 patients with refractory, symptomatic gout, 7 patients were organ transplant recipients. These 7 patients were using a variety of immunosuppressants, including mycophenolate mofetil, cyclosporine, azathioprine, tacrolimus, and cyclosporine. 6 of the 7 patients were able to complete therapy with pegloticase, compared to only 11 of 23 patients who were not receiving immunosuppressive therapies [6].
In a previous paper, we describe the use of methotrexate to allow pegloticase completion in two patients. In these two cases, methotrexate use was able to recapture function of pegloticase when used immediately following a spike in sUA levels, as well as when re-initiated following a lapse in methotrexate compliance. Additionally, Botson-Peterson describe a cohort of 9 patients with refractory tophaceous gout who underwent 4 weeks of methotrexate treatment prior to initiating pegloticase and maintained methotrexate treatment throughout the pegloticase course. 7 of the 9 patients experienced a sharp decrease in sUA (<1.5 mg/ dl) after their first infusion and were able to maintain low sUA levels. The other two achieved low sUA levels after their 2nd and 6th infusions and were able to maintain these levels for the remainder of their treatment period [12].
Patient 1 underwent an additional 6-month course of pegloticase because of residual tophi. After completion of his first 6 months of treatment the patient decided to reduce his methotrexate dosage of 25 mg IM to 15 mg oral due to developing an aversion to self-injection. Response to pegloticase was eventually restored after reinitiating methotrexate 25 mg IM. While various studies [13] have demonstrated the effectiveness of concomitant use of immunosuppressive agents with biologics, Patient 1’s outcome suggests a role for escalating immunosuppressant dosages to recapture pegloticase response. This observation is supported by a study by Krieckaert et al. that demonstrated that methotrexate reduces the formation of antiadalimumab antibodies in a dose-dependent manner [14]. However, the role for increasing dosages of immunosuppressants following the development of anti-drug antibodies, such as in episodes of non-compliance, requires further study. Of note, Patient 5’s methotrexate was reduced from 15 mg to 10 mg due to elevated liver enzymes. Yet, he was able to maintain an adequate response to pegloticase with low sUA throughout his treatment. We speculate that the patient never developed anti-pegloticase antibodies since the initiation of methotrexate; the reduction of methotrexate still provided adequate immunosuppression.
Although Patient 2 was initially treated with methotrexate 15 mg oral, she was switched to azathioprine 50 mg BID due to fatigue; methotrexate is known to cause fatigue in 50- 75% of users, 20% of whom suffer severe fatigue [15]. The use of azathioprine has been previously shown to be successful [9]. Berhanu et al. describe a case of refractory tophaceous gout treated with pegloticase and concomitant low-dose azathioprine, in which pegloticase effectively maintained low serum uric acid levels and was well-tolerated for 98 weeks. Two instances of azathioprine noncompliance were followed by increased sUA levels, but these declined following reinstitution of azathioprine. The benefit of azathioprine use in the formation of anti-drug antibodies, specifically against infliximab and adalimumab, has also been demonstrated in a randomized, double-blind trial by Colombel et al and a multicenter prospective cohort study by Vermeire et al. [16,17].
Patient 7 was given cyclosporine 50 mg BID instead of methotrexate due to elevated liver enzymes caused by non-alcoholic steatosis. Freyne et al. similarly described a heart transplant recipient treated daily with mycophenolate mofetil and cyclosporine who was able to complete a 38-week course of pegloticase infusions without experiencing gout flares or infusion reactions [8].
It appears that multiple immunosuppressants are able to suppress the immune response to pegloticase. Indeed, Bunescu et al. and Tabrizi et al. have previously discussed the effect between anti-proliferative and biologic agents and showed that concomitant use of these medications is associated with higher bioavailability of anti-TNFs [18,19]. We believe that improved outcomes can be achieved by tailoring the specific immunosuppressant used to an individual patient’s needs.
Steroid discontinuation
We report an interesting phenomenon that we believe merits further exploration. According to the drug manufacturer’s guidelines for pegloticase use, IV corticosteroids are indicated prior to each infusion to reduce the possibility of infusion reaction [20]. However, as we gained experience treating patients with refractory gout using pegloticase and concomitant immunosuppressive medications, we discovered that we could lower and eventually discontinue the use of steroids in premedication. We believe this is particularly significant because steroid exposure has significant acute and chronic side effects [21-23].
This was first observed with Patient 2. The high dosage of methylprednisolone 125 mg IV in her premedication exacerbated her diabetes. We elected to decrease her methylprednisolone dosage to 62.5 mg IV on her 2nd infusion and further reduce it to 31.25 mg IV on her 3rd infusion. Methylprednisolone was then discontinued on her 4th infusion; she did not experience any infusion reaction throughout her treatment course.
Owing to our experience with Patient 2, we decreased and ultimately discontinued methylprednisolone in the majority of the patients. The timing of steroid dosage reduction and discontinuation was performed at the administering physician’s discretion. None of the patients experienced infusion reactions after lowering their methylprednisolone dosage.
Overall, these 7 cases support the concomitant use of immunosuppressants with pegloticase to prevent infusion reactions, potentially indicating suppression of anti-pegloticase antibodies. Assuming anti-pegloticase antibodies are adequately suppressed, the necessity of prolonged steroid use in premedication comes into question. Given the many adverse effects of prolonged steroid use and that there have been no instances of infusion reaction in our patients, we believe that steroids may be eliminated as a premedication after the first 2-3 months of infusions, when most infusion reactions occur [24].
This case series has potential limitations. Although the various immunosuppressants used were considered effective and produced similar patient outcomes, cyclosporine and azathioprine were only used in one patient each. This small sample size requires further research into the efficacy of the immunosuppressive medications. Additionally, the timing and collection of sUA levels was inconsistent due to various patient schedules and compliance. Lastly, the timing of the discontinuation of steroids in premedication was performed at the administering physician’s discretion; exact timing of steroid dosage reduction and discontinuation requires further study.
Currently, a prospective study, “Study Of Pegloticase Plus Methotrexate In Patients With Uncontrolled Gout,” is being conducted with Botson and Peterson’s protocol [25]. The study’s goal is to examine the use of immunosuppresive medication administered four weeks prior to treatment with pegloticase. Our cases suggest that immunosuppressive medication may be effectively initiated at the time of the first infusion. Additionally, it seems that immunosuppressive medication may be tailored to a patient’s needs, potentially allowing alteration of the dose, route, or type of medication used. While larger scale studies are warranted to further explore this method, the prospect of reducing exposure to immunosuppressive therapies may have the added benefits of increasing patient compliance, decreasing infection risk, and minimizing potential adverse effects.
Conclusion
Prophylactic use of immunosuppressive therapy with pegloticase may enable sustained treatment and improve outcomes. Concomitant use of immunosuppressive therapy with pegloticase may play a role in preventing infusion reactions, allow reduction of steroids in pre-medication, and result in improved outcomes. We have demonstrated that immunosuppressive therapy may be initiated on the day of the first infusion. Additionally, therapy does not seem to be confined to a single immunosuppressant. Thus, immunosuppressive therapy may be tailored to a patient’s needs. The use of concomitant immunosuppressants with pegloticase treatment warrants further study. Immunosuppressive therapy seems to show the ability to recapture pegloticase response after development of antidrug antibodies. The use of immunosuppressants to prevent anti-drug antibody formation, recapture pegloticase efficacy, and reduce discontinuation rates warrants further study.
Conflicts of Interest
We report no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent for publication of this case report, and any accompanying images, was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Funding support
We declare no financial support.
Availability of data and materials
This is a case report of a single patient, to protect privacy and respect confidentiality; none of the raw data has been made available in any public repository. The original reports, laboratory studies, imaging studies and outpatient clinic records are retained as per normal procedure within the medical records of our institution.
Authors' contributions
FB examined the patient and contributed to manuscript conception, preparation, and editing. SB, MR, MD and FF examined the patient and contributed to manuscript conception, preparation and editing. DR performed pathological readings.
References
- Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC. Med. 15(1), 123–123 (2017).
- Hainer BL, Matheson E, Wilkes RT. Diagnosis, treatment, and prevention of gout. Am. Fam. Physician. 90(12), 831–836 (2014).
- Janssens HJ, Janssen M, van de Lisdonk EH et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 371(9627), 1854–1860 (2008).
- Juraschek SP, Kovell LC, Miller ER et al. Gout, urate-lowering therapy, and uric acid levels among adults in the United States. Arthritis. Care. Res. 67(4), 588–592 (2015).
- Sundy JS, Baraf HSB, Yood RA et al. Efficacy and Tolerability of Pegloticase for the Treatment of Chronic Gout in Patients Refractory to Conventional Treatment: Two Randomized Controlled TrialsPegloticase and Chronic Gout. JAMA. 306(7), 711–720 (2011).
- Hershfield MS, Ganson NJ, Kelly SJ et al. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis. Res. Ther. 16(2), R63 (2014).
- Baraf HS, Yood RA, Ottery FD et al. Infusion-related reactions with pegloticase, a recombinant uricase for the treatment of chronic gout refractory to conventional therapy. J. Clin. Rheumatol. 20(8), 427–432 (2014).
- Freyne B. A Case Report of Immunosuppressant MedicationAssociated Polyarticular Tophaceous Gout Successfully Treated Using the Polyethylene GlycolConjugated Uricase Enzyme Pegloticase. Transplant. Proc. 50(10), 4099–4101 (2018).
- Berhanu AA, Krasnokutsky S, Keenan RT et al. Pegloticase failure and a possible solution: Immunosuppression to prevent intolerance and inefficacy in patients with gout. Semin. Arthritis. Rheum. 46(6), 754–758 (2017).
- https://eurekamag.com/research/066/442/066442724.php
- Bessen SY, Bessen MY, Yung CM. Recapture and improved outcome of pegloticase response with methotrexate-A report of two cases and review of the literature. Semin. Arthritis. Rheum. 49(1), 56–61 (2019).
- Botson J, Peterson J, OPA et al. Pretreatment and Coadministration with Methotrexate Improved Durability of Pegloticase (Krystexxa) Response: A Prospective, Proof-of-Concept, Case Series. American College of Rheumatology Meeting Abstracts. (2018).
- Jani M, Barton A, Warren RB et al. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxford). 53(2), 213–222 (2014).
- Krieckaert CL, Nurmohamed MT, Wolbink GJ. Methotrexate reduces immunogenicity in adalimumab treated rheumatoid arthritis patients in a dose dependent manner. Ann. Rheum. Dis. 71(11), 1914 (2012).
- Curtis JR, Xie F, Mackey D et al. Patient's experience with subcutaneous and oral methotrexate for the treatment of rheumatoid arthritis. BMC. Musculoskelet. Disord. 17(1), 405 (2016).
- Colombel JF, Sandborn WJ, Reinisch W et al. Infliximab, Azathioprine, or Combination Therapy for Crohn's Disease. N. Engl. J. Med. 362(15), 1383–1395 (2010).
- Vermeire S, Noman M, Van Assche G et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut. 56(9), 1226–1231 (2007).
- Bunescu A, Seideman P, Lenkei R et al. Enhanced Fcgamma receptor I, alphaMbeta2 integrin receptor expression by monocytes and neutrophils in rheumatoid arthritis: interaction with platelets. J. Rheumatol. 31(12), 2347 (2004).
- Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug. Discov. Today. 11(1-2), 81–88 (2006).
- KRYSTEXXA. Lake Forest, IL: Horizon Pharma Rheumatology LLC. (2016).
- Rice JB, White AG, Scarpati LM et al. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 39(11), 2216–2229 (2017).
- Liu D, Ahmet A, Ward L et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy. Asthma. Clin. Immunol. 9(1), 30 (2013).
- Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R et al. Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetol. Metab. Syndr. 5, 18 (2013).
- Becker MA, Baraf HSB, Yood RA et al. Long-term safety of pegloticase in chronic gout refractory to conventional treatment. Ann. Rheum. Dis. 72(9), 1469–1474 (2013).
- https://ClinicalTrials.gov/show/NCT03994731