Case Report - Interventional Cardiology (2024) Volume 16, Issue 6
Case report on Friedreich ataxia with coronary artery disease and structural heart disease
- Corresponding Author:
- Ayesha Siddika
Department of Cardiology, United Hospital Limited, Dhaka, Bangladesh,
E-mail: ayeshasiddika835@gmail. com
Received date: 01-Dec-2024, Manuscript No. FMIC-24-157905; Editor assigned: 03-Dec-2024, PreQC No. FMIC-24-157905 (PQ); Reviewed date: 17-Dec-2024, QC No. FMIC-24-157905; Revised date: 23-Dec-2024, Manuscript No. FMIC-24-157905 (R); Published date: 30-Dec-2024, DOI: 10.37532/1755- 5310.2024.16(6).940
Abstract
Approximately 50% of patients with Friedreich's ataxia exhibit cardiomyopathy on echocardiography, making it the most prevalent genetic neurodegenerative condition. Since coronary artery disease has historically been discounted, even when angina has been documented in these people, its significance is understated. Angiographically, patients with Friedreich's ataxia have rarely shown evidence of premature obstructive coronary disease. Here, we present an unusual case of a 35-year-old man with Friedreich's ataxia who presented with severe central compressive chest pain associated with dyspnea and diaphoresis and was finally diagnosed as a case of acute myocardial infarction of the anterior wall. Due to the likelihood of underdiagnosing coronary artery disease in this population, this case highlights the significance of thoroughly examining anginal symptoms in individuals with Friedreich's ataxia. An aggressive treatment plan and a delay in the development of end-stage cardiomyopathy may be made possible by an early diagnosis.
Hypertrophic cardiomyopathy • Frataxin • Friedreich ataxia • Echocardiogram
Introduction
A genetic mutation causing a disruption in the frataxin gene results in Friedreich Ataxia (FA), an autosomal recessive neurological illness. Roughly 1 in 50,000 white people have this rare illness [1-3].
There is no doubt that myocardial involvement occurs in FA; the most common cardiac finding is either concentric or, less commonly, asymmetrical Left Ventricular (LV) hypertrophy [4-6]. In a retrospective analysis of 99 individuals with FA, cardiac dysfunction-which predisposes to congestive heart failure and supraventricular arrhythmias-was the most common cause of mortality (59%) [7].
Case Presentation
A 35-year man diabetic, normotensive and unmarried presented to our emergency department with complaints of central compressive chest pain associated with sweating and left arm radiation for 2 days. An examination of this patient revealed that pulse 84 beats/min, BP-100/60 mmHg, Heart-S1 and S2 audible there was no added sound and both lung bases were clear. Neurological examination revealed that the patient was ataxic, dysarthric, had loss of coordination, hearing impairment and complete physical disability. He had a history of Friedreich ataxia for the last 15 years and his elder sister had the same type of illness.
As the patient had typical chest pain so cardiac evaluation was done. ECG findings revealed ST elevation and symmetrical T wave inversion in all precordial leads as shown in (Figure 1). Troponin I was highly raised 64.23 ng/mL, CK MB-220 U/L. Other haematological parameter revealed Hb%: 12.8 gm/dL, WBC: 5,900/mm3, Polymorph: 48%, Lymphocyte: 44%, S. Creatinine: 1.8 mg/dL, Urea: 28 mg/ dl, RBS: 7.0 mmol/L, HbA1c: 7.0% and S.electrolyte: Na/k/Cl- 144/3.30/100.8 mmol/L. Blood grouping: O+ve. HBsAg: –ve, Anti HCV: Negative, Anti HIV 1 and 2: Negative.
Evaluation of the patient's lipids profile revealed total cholesterol of 158 mg/dL, triglycerides of 100 mg/dL, high-density lipoprotein cholesterol of 38 mg/dL and low-density lipoprotein cholesterol of 88 mg/dL.
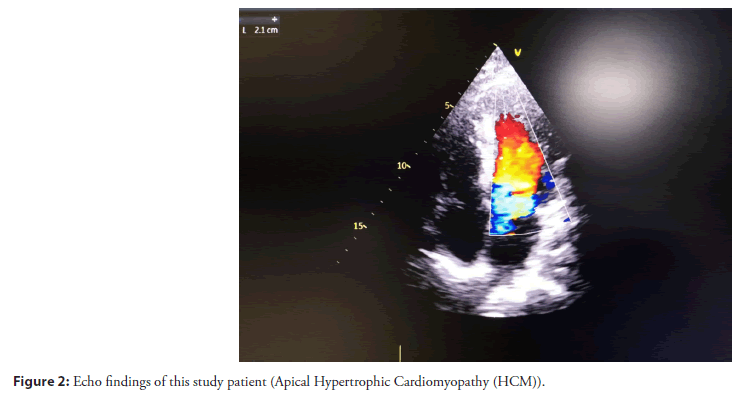
An echocardiogram was done for cardiac involvement especially to see the regional wall motion abnormality and exclusion of cardiomyopathy. So, echocardiogram findings revealed that an apical variety of hypertrophic cardiomyopathy and associated with regional wall motion abnormality, LVEF: 40%-42% as shown in (Figure 2).
Figure 2: Echo findings of this study patient (Apical Hypertrophic Cardiomyopathy (HCM)).
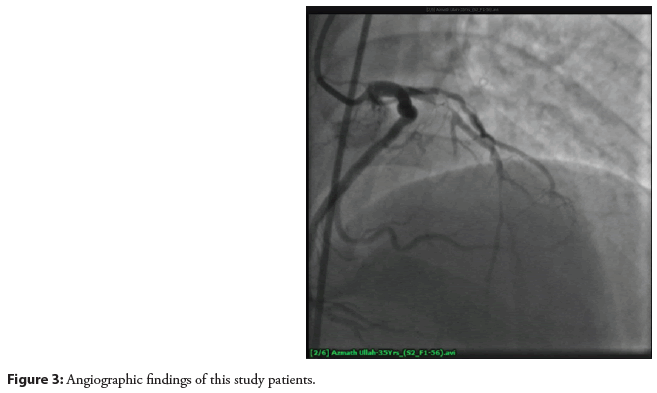
So as per treatment protocol and evaluation strategy, a coronary angiogram was done and revealed 90%-95% stenosis in its proximal segment followed by 60%-65% stenosis in its mid midsegment. So, the recommendation was per cutenous cutaneous coronary intervention to the left anterior descending arteries as shown in (Figure 3).
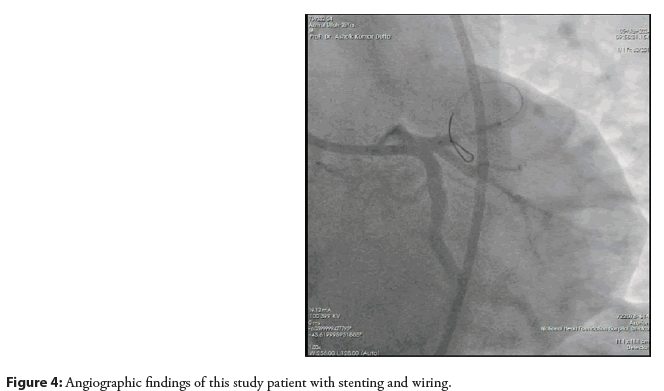
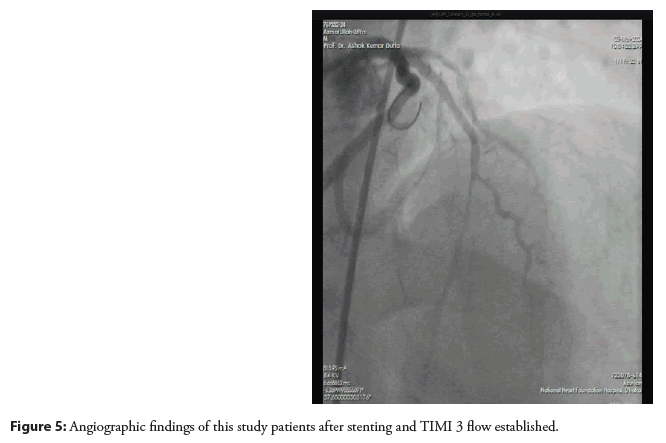
So, after predilation covering the lesion stent (XIENCEPRIME- 3.00 × 18 mm) was deployed. The final angiogram showed the vessel was well revascularized with TIMI-III distal flow as shown in (Figures 4 and 5).
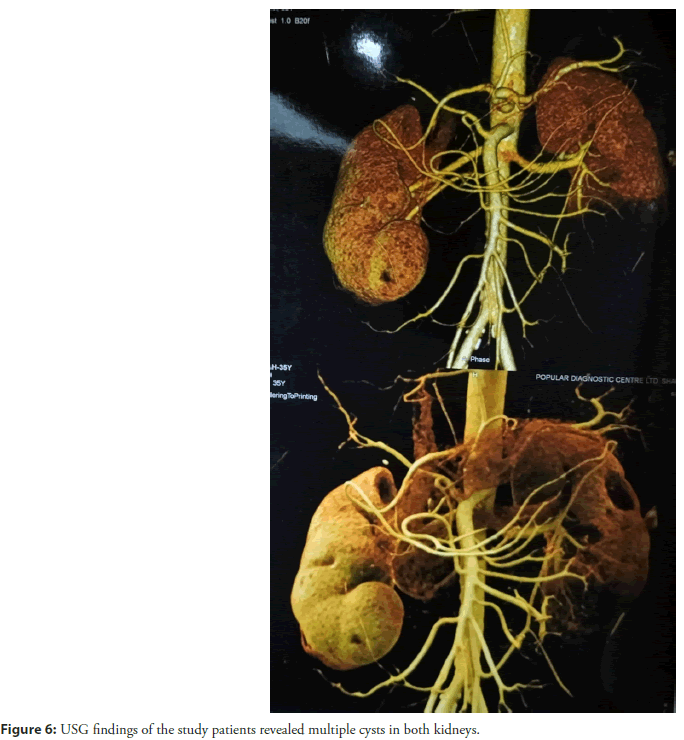
Ultrasonography (USG) of the whole abdomen with special attention to both kidney regions revealed multiple cystic lesions in both kidneys highest measuring about 3.1 × 3.2 as shown in (Figure 6).
Results and Discussion
Professor Nikolaus Friedreich of the University of Heidelberg first recognized the illness in the 1860s when he reported two affected siblings with asymptomatic parents [8]. The first published set of diagnostic criteria based on clinical symptoms was released in 1976. Until 1996, the revised criteria reported by Harding et al., in 1981 were the preferred diagnostic tool [9]. This changed when Campuzano et al., discovered the causal mutation in the frataxin gene in 1996, making genetic testing possible [10]. The diagnosis of FA frequently depends on ruling out acute neurologic abnormalities and observing a gradually progressing clinical course, even in the age of genetic testing. Consequently, diagnosis is frequently postponed. Ataxia of the limbs and trunk, dysarthria, diabetes mellitus and heart disorders are among the clinical symptoms of FRDA. When there are questionable clinical features, the diagnosis is first made.
A positive Babinski reflex, pes cavus, scoliosis and cardiomyopathy were the secondary criteria, which were first described by Harding et al., [9]. The primary criteria included progressive gait and limb ataxia, absent patellar and ankle reflexes, dysarthria, muscle weakness, loss of vibration or position and onset before the age of 25. So this matched with our study patient. Our patient manifestation started 15 years ago but at the age of 35 years now he presented with premature coronary artery disease in the form of acute myocardial infarction. This full scenario repetition happened with his elder sister and patient died at the age of 38 years.
Siddika et al., showed in their study among a total of 140 pre and post-menopausal women with ACS and described that the pre-menopausal women who had a history of premature coronary artery disease were more suffering from severe coronary artery disease [11-13].
But some studies state that Friedreich's ataxia patients have been known to experience angina, albeit no link has been shown between the condition and coronary heart disease [14,15].
As far as we are aware, the literature from around the world has only reported 4 instances of acute myocardial infarction related to Friedreich's ataxia [16].
For this young patient, Friedreich's ataxia cannot be ruled out as a possible cause of the development of early epicardial coronary disease, even though it may be related to his usual coronary risk profile. That other conventional cardiac risk factors have an exponentially increased effect due to the condition is also highly likely. In the instance studied by Sharma and colleagues, the possibility that Friedreich's ataxia caused coronary artery disease was brought up, but they doubted it because their patient also had dyslipidemia and hyperhomocysteinemia, which they believed were major factors in the disease.
In patients suffering from Friedreich's ataxia, it is important to recognize conventional risk factors and start vigorous medical treatment right away. Though it is not a commonly reported cause of death, sudden cardiac death is possible.
Conclusion
A young patient with Friedrich ataxia with typical chest pain must be evaluated properly though the ECG findings are almost similar to hypertrophic cardiomyopathy. So premature coronary artery disease suffering from Friedreich ataxia is not uncommon. A thorough clinical examination and some investigation must be required. To obtain additional information, a comprehensive community-based study should be conducted. Raising social awareness about primary and secondary prevention, as well as early detection and treatment, is essential to lowering the morbidity, mortality and overall burden of premature coronary artery disease related worse outcome.
References
- Santos R, Lefevre S, Sliwa D, et al. Friedreich ataxia: Molecular mechanisms, redox considerations, and therapeutic opportunities. Antioxid Redox Signal. 13(5):651-690 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Pandolfo M, Pastore A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J Neurol. 256:9-17 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Pandolfo M. Friedreich ataxia: The clinical picture. J Neurol. 256:3-8 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Schulz JB, Boesch S, Bürk K, et al. Diagnosis and treatment of Friedreich ataxia: A European perspective. Nat Rev Neurol. 5(4):222-234 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Dürr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 335(16):1169-1175 (1996).
[CrossRef] [Google Scholar] [PubMed]
- Mottram PM, Delatycki MB, Donelan L, et al. Early changes in left ventricular long-axis function in Friedreich ataxia: Relation with the FXN gene mutation and cardiac structural change. J Am Soc Echocardiogr. 24(7):782-789 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Tsou AY, Paulsen EK, Lagedrost SJ, et al. Mortality in Friedreich ataxia. J Neurol Sci. 307(1-2):46-49 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Hewer RL. The heart in Friedreich's ataxia. Br Heart J. 31(1):5 (1969).
[CrossRef] [Google Scholar] [PubMed]
- Harding AE. Friedreich's ataxia: A clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 104(3):589-620 (1981).
[CrossRef] [Google Scholar] [PubMed]
- Campuzano V, Montermini L, Lutz Y, et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet. 6(11):1771-1780 (1997).
[CrossRef] [Google Scholar] [PubMed]
- Siddika A , Malik F, Kalimuddin M, et al. Sociodemographic, clinical and angiographic overview of women with acute coronary syndrome : A current prospective hospital based study in Bangladesh. 5(1):1-10 (2024).
- Siddika A, Kalimuddin M, Hasan N, et al. Severity of coronary artery disease by friesinger score among women with acute coronary syndrome in a tertiary care hospital in Bangladesh. Cureus. 16(2) (2024).
[CrossRef] [Google Scholar] [PubMed]
- Siddika A, Kalimuddin M, Hasan N, et al. Severity of coronary artery diseases among pre-and postmenopausal women with acute coronary syndrome: A hospital-based study in Bangladesh. Cureus. 15(12) (2023).
[CrossRef] [Google Scholar] [PubMed]
- Albano LM, Nishioka SA, Moysés RL, et al. Friedreich's ataxia: Cardiac evaluation of 25 patients with clinical diagnosis and literature review. Arq Bras Cardiol. 78(5):448-451 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Hewer RL. The heart in Friedreich's ataxia. Br Heart J. 31(1):5 (1969).
[CrossRef] [Google Scholar] [PubMed]
- Sharma AK, Kiyokawa M, Kim ET, et al. Acute myocardial infarction and Friedreich's ataxia. Hawaii Med J.61(9):199-201 (2008).
[Google Scholar] [PubMed]