Case Report - Clinical Investigation (2022) Volume 12, Issue 3
Attack on the heart: Hydroxychloroquine cardiotoxicity induced dysrhythmia and cardiomyopathy
- Corresponding Author:
- Tom Hu
Department of Cardiology, Oklahoma State University, Tulsa, Oklahoma, USA
E-mail: tom.hu@okstate.edu
Received: 05-March-2022, Manuscript No. fmci-22-56167; Editor assigned: 07-March-2022, PreQC No. fmci-22-56167 (PQ); Reviewed: 15-March-2022, QC No. fmci-22-56167 (Q); Revised: 16-March-2022, Manuscript No. fmci-22-56167 (R); Published: 24-March-2022; doi: 10.37532/2041-6792.2022.12(3).54-60
Abstract
Hydroxychloroquine (HCQ) induced cardiotoxicities are rare but significant complications after prolonged HCQ use with either cardiomyopathy or conduction abnormalities. We present a case of a 70-year-old female with mixed connective tissue disease on long term HCQ use presenting with decompensated heart failure with reduced ejection fraction and symptomatic heart block.
Keywords
Hydroxychloroquine • Cardiotoxicity • Cardiomyopathy • Dysrhythmia
Case Presentation
History of Presentation
70-year-old female presented with shortness of breath and fatigue. Her symptoms had been ongoing for approximately two days. Patient had a syncopal episode walking back from the bathroom. Her heart rate was in the low 30’s upon EMS arrival. At the emergency department, she continued to experience dizziness. Her vital signs on presentation were heart rate 33 bpm, respiratory rate 16 breaths/min, blood pressure 167/78 mmHg, temperature 36.1°C, SpO2 98%, Height 157.5 cm, Weight 149 kg. Physical examination revealed mild JVD, bradycardic heart rate without significant murmur, bibasilar rales, and 2+ pre-tibial edema.
Past medical history
The patient’s past medical includes essential hypertension, chronic kidney disease stage IIIa, morbid obesity, diabetes mellitus type II, mixed connective tissue disease. Her current medications included amlodipine 2.5 mg daily PO, aspirin 325 mg daily PO, atorvastatin 20 mg QHS PO, hydrochlorothiazide 25 mg daily PO, hydroxychloroquine 400 mg BID PO, Levemir 15 units daily subcutaneously, losartan 100 mg daily PO.
Differential Diagnosis
The differential diagnosis of patient’s dyspnea, fatigue and syncope is broad including cardiac, pulmonary, hematologic cases such as coronary artery disease, cardiomyopathy, arrhythmias, valvopathy, obstructive pulmonary disease, anemia.
Investigations
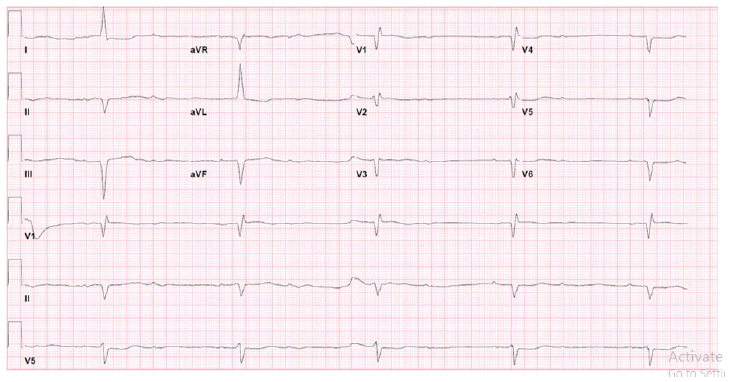
The electrocardiogram showed showed 2:1 AV block with left ventricular hypertrophy and non-specific interventricular conduction delay (Figure 1).
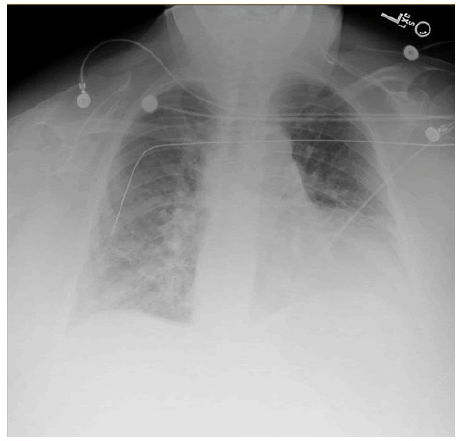
Single-view chest X-ray showed mild cardiac enlargement and perivascular congestion suggestive of pulmonary edema (Figure 2).
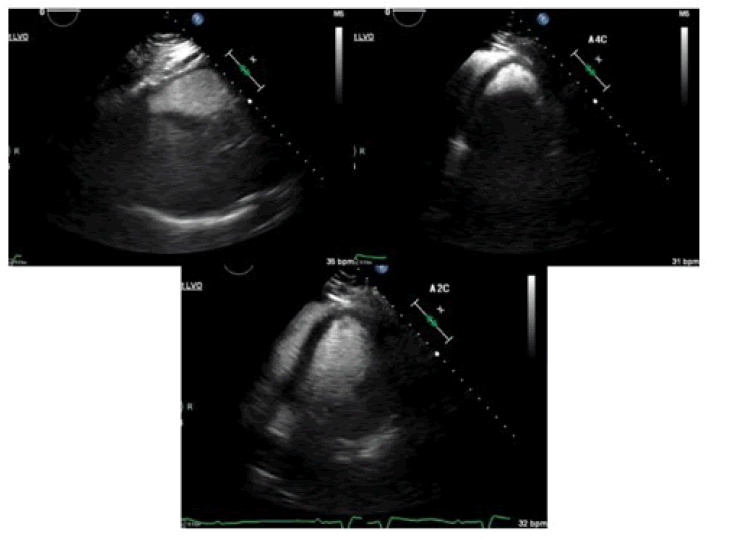
Laboratory results showed creatinine 1.39, GFR 38, troponin <0.05, BNP 1,061, hemoglobin 12.7, white blood cell count 8.8, and platelet count 173,000. Patient was admitted for her 2:1 AV block. Echocardiogram showed LVEF 30% with severe global hypokinesis, moderately dilated LV cavity, mild left ventricular hypertrophy, mild bi-atrial dilation, mild mitral regurgitation, and dilated IVC without collapse (Figure 3 and Videography Image 1).
Figure 3: Echocardiogram and showed LVEF 30% with severe global hypokinesis, moderately dilated LV cavity, mild left ventricular hypertrophy, mild bi-atrial dilation; (A): Parasternal long-axis view end diastolic; (B): Parasternal long-axis view end systolic; (C): Apical 2 chamber view end diastolic; (D): Apical 2 chamber view end systolic.
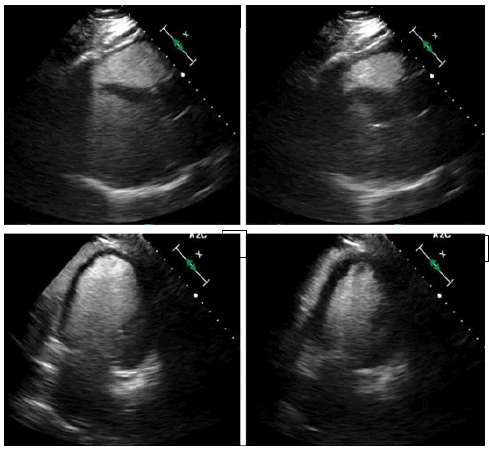
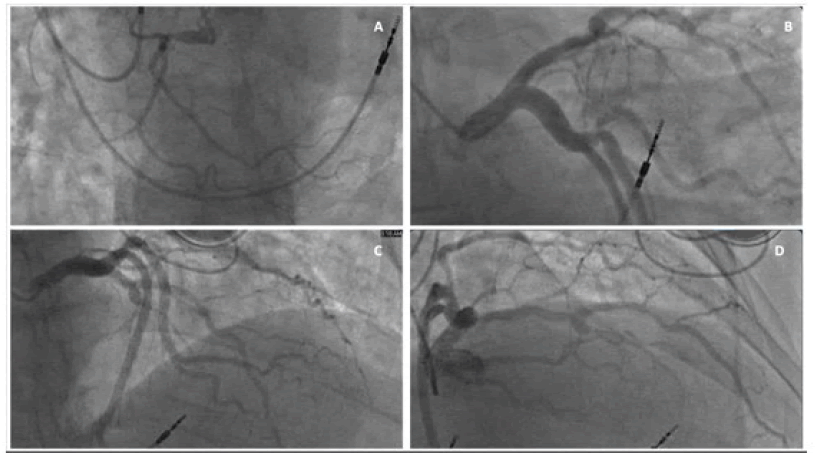
Left heart catheterization revealed 70% stenosis of mid Left Anterior Descending artery (LAD), mild disease in Left Circumflex artery (Cx), and no significant disease in the small non-dominant Right Coronary Artery (RCA) (Figure 4 and Videography Image 2).
Figure 4: Coronary angiographic image demonstrating RCA is small, non-dominant with no significant stenosis. LAD has a 70% stenosis at mid portion with calcification, Cx showed mild luminal irregularities; (A): RAO-CRA view of RCA; (B): LAO-CAU view of Cx; (C): LAO-CRA view of LAD; (D): RAO-CRA view of LAD.
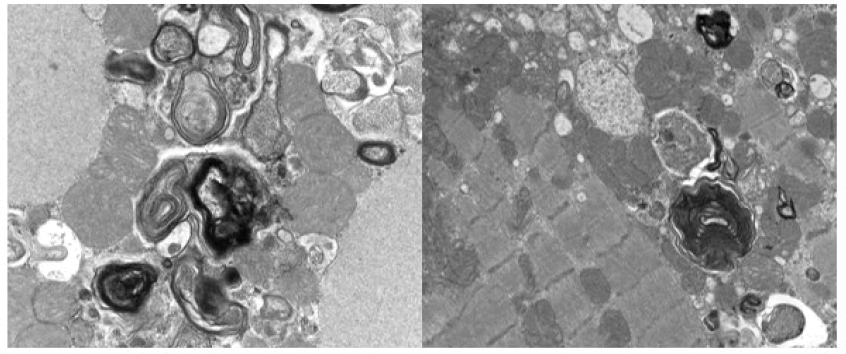
An interdepartmental discussion was held regarding her cardiomyopathy is out of proportion to her coronary artery disease and intervention of the mid LAD was deferred. Given her long-term use of hydroxychloroquine for 5-10 years prior to presentation, dilated cardiomyopathy, and 2:1 AV block it was felt Endomyocardial Biopsy (EMB) would be warranted to further investigate her cardiomyopathy. Preliminary results from EMB with light microscopy showed no evidence of inflammatory infiltrates or myocarditis and Congo red stain was negative. The transmission electron microscopic samples revealed scattered myelinoid and curvilinear bodies contained within myocytes consistent with hydroxychloroquine induced cardiomyopathy (Figure 5).
Figure 5: Transmission electron microscopic images of right ventricular endomyocardial biopsy revealing myelinoid and curvilinear bodies consistent with hydroxychloroquine induced cardiomyopathy.
Management
Given her continued symptoms, decision was made to proceed with dual chamber permanent pacemaker implantation for her symptomatic 2:1 AV block absence of chest pain with a cardiomyopathy out of proportion to the coronary artery disease.
She was successfully implanted with a dual chamber permanent pacemaker and started on diuretic. Over two days from device implantation the patient showed improvement in her shortness of breath, lower extremity edema, and renal function. She was able to be placed on guideline directed medical therapy for her cardiomyopathy.
Rheumatology was consulted and recommended changing her medications for mixed connective tissue disease to azathioprine 100 mg daily.
Discussion
Hydroxychloroquine is derived from chloroquine with an additional hydroxyl group at the end of the side change and has significantly decreased toxicity. Hydroxychloroquine has long been a stable of connective tissue disease chronic treatment. Hydroxychloroquine toxicity is rare with incident of retinopathy at 0.3% and neuromyopathy at 0.19% [1-3]. Hydroxychloroquine’s cardiotoxicities however are difficult to assess due to the lack of systematic studies with many of the pathologically confirmed cases reported as case reports. A previous systematic review demonstrated a dose-dependent relationship with hydroxychloroquine and its cardiotoxicities. It takes, on average, seven years, and a cumulative dose of 1200 g to develop cardiotoxicities [1,4]. The prominent side effect was conduction disorders, followed by ventricular hypertrophy, hypokinesis, heart failure, pulmonary hypertension, and valvular dysfunction. Acute toxicity is primarily related to hydroxychloroquine Vaughan William Class IA antiarrhythmic effect like quinidine. Hydroxychloroquine exerts its antiarrhythmic effect by inhibiting voltage-gated sodium and potassium channels leading to increased QRS and QTc intervals [5]. Several risk factors increase the QTc prolongation, such as female gender, preexisting structure heart disease, electrolyte imbalance, particularly with hypokalemia, both acquired and inherited QTc prolongation conditions. Chronic toxicity involves disrupting the lysosomal degradation pathway leading to accumulation of the metabolites, myofiber necrosis, and mitochondrial damage. In addition, the accumulation of the metabolites can directly affect the conduction system and the cardiac myocytes [5].
The typical presentation of chronic hydroxychloroquine conduction toxicity includes bundle branch blocks and variable heart block. Frequently the conduction disorder can be mistakenly attributed to the underlying connective tissue disease that hydroxychloroquine is being used to treat. The conduction disorders can be sequential, with the right bundle branch affected first, followed by the left bundle branch, then ultimately manifests as complete heart block [4].
The similarity between Anderson-Fabry disease and hydroxychloroquine toxicity suggests lysosomal dysfunction plays an integral part in the conduction system. Hydroxychloroquine is a weak cation that can cross the cell membrane and accumulate in lysosomes. By directly inhibiting phospholipase and decreasing lysosomal hydrolase this allows for accumulation of metabolic byproducts and disruption of autophagy [4].
Biventricular hypertrophy is the most common finding on cardiac imaging. In addition, cardiac MRI can demonstrate the extent of fibrosis by late gadolinium enhancement. Cardiac MRI with T1 mapping is sensitive to diagnosing Anderson-Fabry disease. Given the similarity of Anderson- Fabry disease and HCQ induced cardiomyopathy, it is reasonable to infer that cardiac MRI can be sensitive for hydroxychloroquine-induced cardiomyopathy. To differentiate the two diagnoses would require detailed history and ultimately endomyocardial biopsy results [4,6].
The definitive diagnosis of hydroxychloroquine-induced cardiomyopathy is made with electron microscopy demonstrating myeloid bodies and curvilinear inclusion bodies [4,7]. Myeloid bodies are the inclusions of accumulated glycolipids and glycoproteins due to the decreased α-galactosidase A activity. This can be seen in both Anderson-Fabry disease and hydroxychloroquine toxicity. Curvilinear inclusions, known for their “commashaped” structures, are autophagolysosome with poorly digested mitochondrial ATP synthases. Curvilinear bodies are not present in Anderson-Fabry disease, which helps differentiate hydroxychloroquine-induced cardiomyopathy from Anderson-Fabry disease.
Despite previous reports and having a similar proportional reporting ratio for cardiomyopathy as Trastuzumab and Doxorubicin, hydroxychloroquine-induced cardiotoxicities have been underdiagnosed. Clinical guidelines from rheumatological societies continue to recommend HCQ as one of the first-line disease-modifying antirheumatic drugs or as a part of the treatment for connective tissue disease. There is structured screening for macular damages but not for cardiomyopathy. Given its prevalence in usage, it can be assumed that hydroxychloroquine related cardiomyopathy is undiagnosed due to its variable clinical presentation and confounding comorbidities [8,9]. Current treatment for the disease requires a high clinical suspicion and early diagnosis with abrupt withdrawal of hydroxychloroquine. At the early stages of the disease, abrupt withdrawal with standard heart failure therapy may prevent the progression of the disease and allow for some recovery in LVEF, although the complete resolution has not been described. If Hydroxychloroquine toxicity is confirmed the patient should be switched to other antirheumatic agents to help with treatment of their rheumatologic condition [10].
Follow-up
The patient has since been seen in heart failure clinic. She reports being able to ambulate about 70 feet before needing to stop due to fatigue. She has had improvement in her lower extremity edema and is scheduled to undergo a repeat echocardiogram to reevaluate her LVEF in approximately 30 days for consideration of upgrade of her device to an ICD and consideration for cardiac resynchronization therapy.
Conclusion
Cardiotoxicity of hydroxychloroquine is associated with long-term exposure of the agent and is frequently underdiagnosed. Due to the lack of systematic studies, there is no approved therapy to date to treat the cardiotoxicity, outside of discontinuation of the drug. A high level of clinical suspicion in the at-risk population is critical to make the diagnosis complimented with cardiac imaging and endomyocardial biopsy with electron microscopy. Once confirmed, the patient should be switched to other antirheumatic agents and receive standard therapy for congestive heart failure.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Guedira N, Hajjaj-Hassouni N, Srairi JE, et al. Third-degree atrioventricular block in a patient under chloroquine therapy. Rev Rhum Engl Ed. 65:58-62 (1998).
Google Scholar Crossref - Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Ann Rheum Dis. 69:20-28 (2010).
Google Scholar Crossref - Wang C, Fortin PR, Li Y, et al. Discontinuation of antimalarial drugs in systemic lupus erythematosus. J Rheumatol. 26:808-815 (1999).
Google Scholar Crossref - Yogasundaram H, Putko BN, Tien J, et al. Hydroxychloroquine-induced cardiomyopathy: Case report, pathophysiology, diagnosis, and treatment. Can J Cardiol. 30:1706-1715 (2014).
Google Scholar Crossref - Ahmadizar F, Soroush N, Ikram MA, et al. QTc-interval prolongation and increased risk of sudden cardiac death associated with hydroxychloroquine. Eur J Prev Cardiol. 28:1875-1882 (2022).
Google Scholar Crossref - Tönnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy- A review of the literature. Immunopharmacol Immunotoxicol. 35:434-442 (2013).
Google Scholar Crossref - Nadeem U, Raafey M, Kim G, et al. Chloroquine- and Hydroxychloroquine- induced cardiomyopathy: A case report and brief literature review. Am J Clin Pathol. 155:793-801 (2021).
Google Scholar Crossref - Armstrong WF, Ryan T. Feigenbaum's echocardiography. Wolters Kluwer 8th Edi (2019).
Google Scholar Crossref - Ratliff NB, Estes ML, Myles JL, et al. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N Engl J Med. 316:191-193 (1987).
Google Scholar Crossref - Abbi B, Patel S, Kumthekar A, et al. A case of cardiomyopathy with long-term Hydroxychloroquine use. J Clin Rheumatol. 26:e300 (2020).
Google Scholar Crossref