Review Article - Interventional Cardiology (2015) Volume 7, Issue 5
An update on drug-eluting stents versus bare-metal stents in PCI treatment: are there any remaining indications for BMS use?
- Corresponding Author:
- Bernhard Meier
Department of Cardiology, Bern University Hospital, 3010 Bern, Switzerland
E-mail: bernhard.meier@insel.ch
Abstract
Drug-eluting stents (DES) are recognized as a breakthrough technology in the treatment of coronary artery disease, primarily due to substantial reduction in the risk of repeat revascularization as compared with bare-metal stents (BMS). During the last decade new DES – with thinner stent struts, novel durable or biodegradable polymer coatings, and new limus antiproliferative agents – have been developed to address the limitations of earlier generation DES. Randomized trials, observational studies and meta-analyses have shown a marked improvement in clinical safety and efficacy with new-generation DES as compared with BMS, as well as early-generation DES, in a wide spectrum of patients. The objective of this article is to review whether there are any indications left for the use of BMS in clinical practice based on available evidence.
Keywords
bare-metal stents, coronary artery disease, coronary revascularization, coronary stents, drug-eluting stents
Drug-eluting stents (DES) are recognized as breakthrough technology percutaneous coronary interventions (PCI) for the treatment of coronary artery disease [1]. They were proposed in the late 90s to address the issue of restenosis observed in 20 to 30% of patients treated with bare-metal stents (BMS) [2]. Durable polymer-based sirolimus-eluting stents (SES; Cypher®, Cordis, Johnson & Johnson, FL, USA) were first implanted in humans in 1999 and showed a marked reduction of restenosis (0 vs 27%) in the RAVEL (Randomized study with the sirolimus-eluting Bx velocity balloon-expandable stent in the treatment of patients with de novo native coronary artery lesions) study of patients with simple lesions randomly assigned to treatment with SES or BMS [3]. SES were rapidly followed by the introduction of durable polymer-based paclitaxel-eluting stents (PES; Taxus®, Boston Scientific, MA, USA), which were also shown to reduce restenosis and the need for repeat revascularization procedures compared with BMS [4,5].
A large number of randomized trials consistently reported improved clinical outcomes with DES as compared with BMS, primarily due to substantial reduction in the risk of repeat revascularization [6,7]. In 2006, several reports called into question the long-term safety of DES – specifically with respect to the risk of stent thrombosis (ST) [8–11]. Early-generation SES and PES were shown to be associated with a small risk of ST during the followup period beyond 1 year of stent implantation, after discontinuation of dual antiplatelet therapy (DAPT). It was noted, however, that this small excess in ST risk did not translate into a higher risk of death and myocardial infarction [6,7,10,12,13]. During the last decade, new generations of DES have been developed in order to address the limitations of earlier generation SES and PES. Numerous randomized trials [14–17], meta-analyses [18–22], and large-scale registries [23–25] have shown a marked improvement in clinical safety and efficacy with new-generation DES as compared with early-generation DES and BMS in a wide spectrum of patients. The objective of this article is to review whether there are any indications left for the use of BMS in clinical practice based on available evidence.
Why are we still using bare-metal stents?
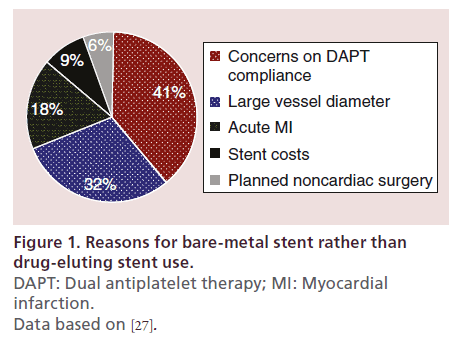
In contemporary clinical practice, BMS are still used in 20–30% of PCI procedures [26]. A recent observational study investigated the reasons for BMS implantation in a cohort of 774 consecutive patients with coronary artery disease undergoing PCI at 31 European centers over a period of 2 months in 2012 [27]. As displayed in Figure 1, the most frequent reason for BMS use was related to concerns on DAPT compliance. This was followed by the presence of a target vessel with a large reference diameter, treatment of patients with acute myocardial infarction, concerns related to reimbursement and planned noncardiac surgery within 1 year after stent implantation.
Figure 1. Reasons for bare-metal stent rather than
drug-eluting stent use.
DAPT: Dual antiplatelet therapy; MI: Myocardial
infarction.
Data based on [27].
Concerns on dual antiplatelet therapy compliance
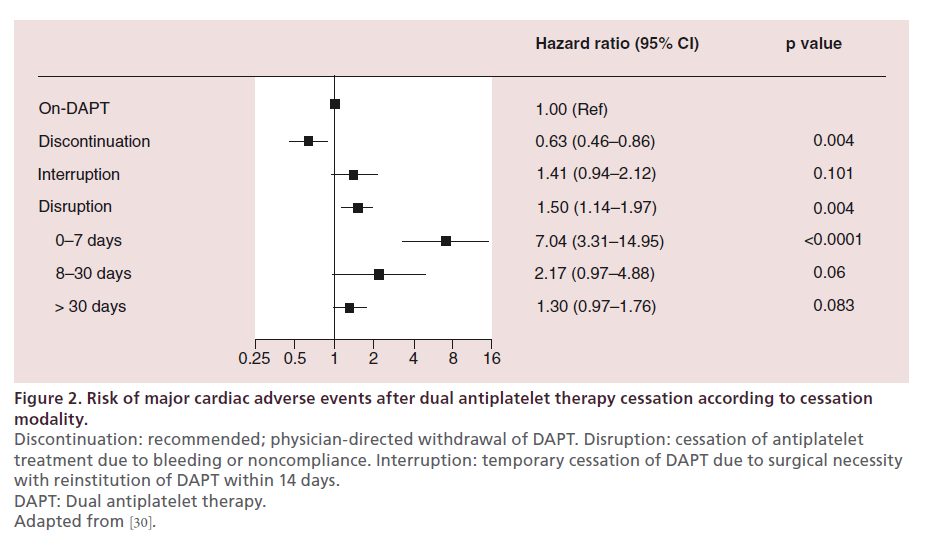
Several observational investigations have associated premature DAPT cessation after PCI with an increased risk of ischemic events [28,29]. Since DAPT is recommended for a longer duration after DES implantation than BMS implantation, the potential lack of compliance to DAPT of patients treated with DES represents a matter of concern for many physicians. These concerns were raised by the evidence of an increased risk of very late ST with early-generation DES [11]. It is notable, however, that the risk of ST at any time and irrespective of DAPT with newer DES is comparable if not smaller than the risk of ST with BMS [18,19,21]. The recently published PARIS (Patterns of nonadherence to antiplatelet regimens in stented patients: an observational single-arm study) registry including 5018 patients treated with stent implantation has confirmed a higher risk of ischemic events after DAPT disruption, intended as nonphysician-directed premature cessation of DAPT (Figure 2) [30]. It is noteworthy that a sensitivity analysis of the same registry indicated that the risk of ischemic events after DAPT disruption appeared not to be influenced by the type of stent initially implanted (i.e., DES vs BMS).
Figure 2. Risk of major cardiac adverse events after dual antiplatelet therapy cessation according to cessation
modality.
Discontinuation: recommended; physician-directed withdrawal of DAPT. Disruption: cessation of antiplatelet
treatment due to bleeding or noncompliance. Interruption: temporary cessation of DAPT due to surgical necessity
with reinstitution of DAPT within 14 days.
DAPT: Dual antiplatelet therapy.
Adapted from [30].
Moreover, the findings of a pooled analysis of four prospective multicenter trials including 4896 patients treated with new-generation zotarolimus-eluting stents suggested that DAPT cessation as early as 1 month may be safe after implantation of a new-generation DES [31]. The findings of this single observational investigation should be regarded as hypothesis generating and will require confirmation in larger randomized trials.
Taken together, available evidence indicates that concerns on DAPT compliance should not prevent from the use of DES in patients undergoing PCI and even suggests that ST with DES might be less frequent than with BMS on any type of antiplatelet treatment in the first year and not more frequent in the subsequent years.
Large vessel diameter
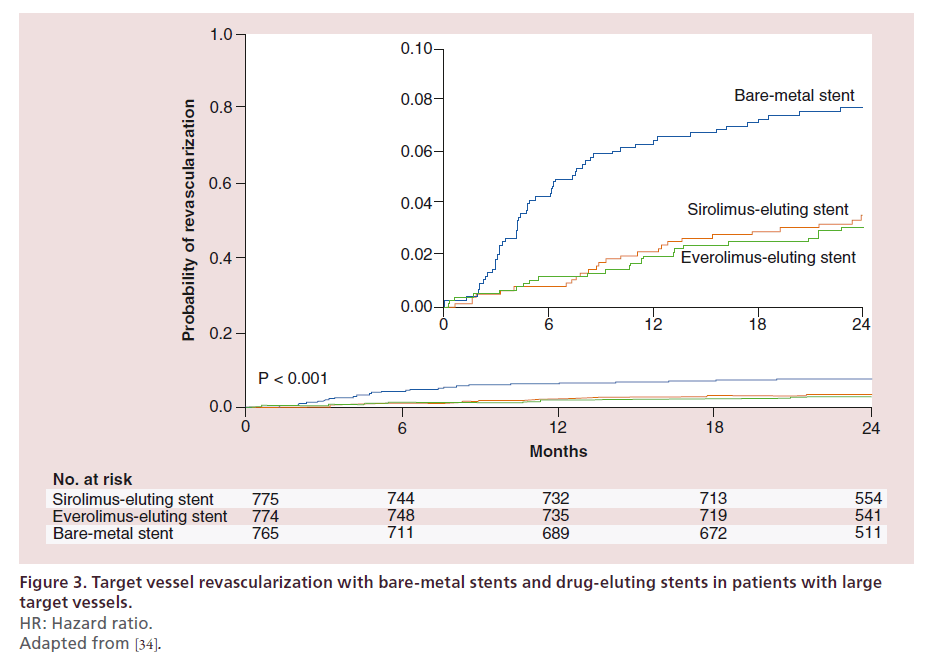
The benefits of DES have been considered uncertain in patients with target lesions located in large coronary arteries. This was based on data indicating a reduced antirestenotic superiority of DES over BMS in coronary arteries with a reference diameter equal or above 3 mm as compared with the effect observed in smaller coronary arteries [32,33]. It is not surprising, therefore, that the large diameter of the target vessel appears as one of the reasons for BMS implantation in clinical practice. However, the hypothesis of only negligible advantage of DES in large coronary arteries was confuted by the BASKET-PROVE trial [34]. In this trial, the use of early-generation SES and new-generation everolimus-eluting stents (EES) was compared with BMS in large coronary arteries of all-comer patients with significant coronary artery disease not requiring oral anticoagulation and not scheduled to undergo surgery. The primary end point of the BASKET-PROVE trial, the composite of death and myocardial infarction, did not differ significantly between patients treated with early-generation SES, new-generation EES and BMS during 2 years of follow-up. However, it is noteworthy that patients treated with DES (i.e., pooled SES and EES) had a significantly lower risk of the composite of death and myocardial infarction as compared with patients treated with BMS (risk ratio 0.60, 95% CI: 0.39–0.93). In addition, the BASKET-PROVE showed a relative risk reduction of approximately 56% in terms of repeat revascularization with DES as compared with BMS (risk ratio 0.44, 95% CI: 0.31–0.63) (Figure 3). Similar findings were confirmed in the recently published BASKET-PROVE II trial, comparing new-generation biolimus-eluting stents (BES) with EES and BMS [35]. In view of the now proven superiority of DES compared with BMS in large coronary arteries, it appears unreasonable to consider the presence of a target vessel with a large reference diameter a valid reason for BMS implantation.
Figure 3. Target vessel revascularization with bare-metal stents and drug-eluting stents in patients with large
target vessels.
HR: Hazard ratio.
Adapted from [34].
ST-segment elevation myocardial infarction
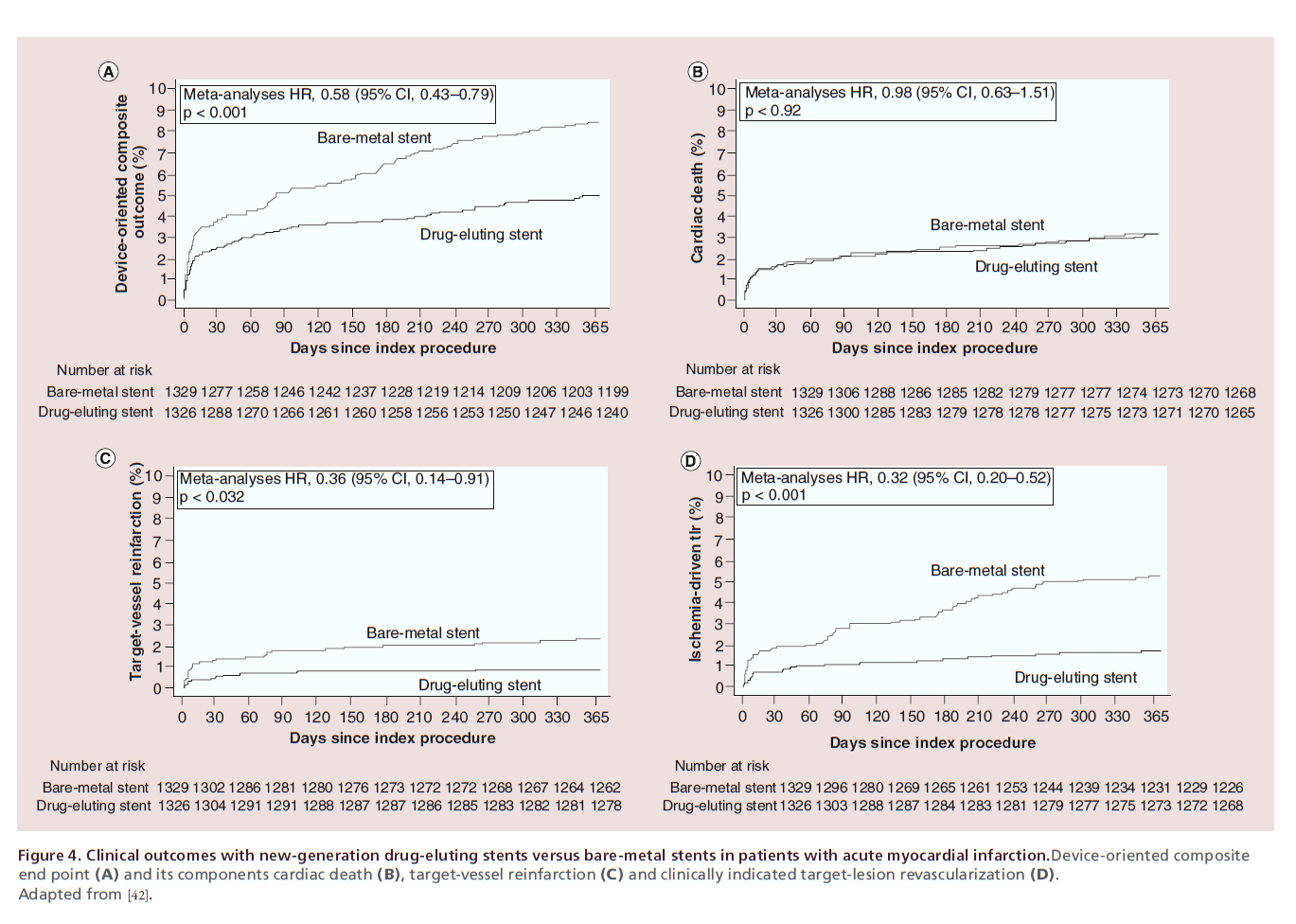
Early-generation DES have been shown superior to BMS in terms of device effectiveness in patients with acute myocardial infarction (MI), with no safety concern [36,37]. Nevertheless, the thrombogenic milieu coupled with evidence of delayed arterial healing due to chronic inflammation – with persistent fibrin deposition, positive vessel remodeling and uncovered stent struts – following early-generation DES implantation in acute MI culprit lesions have led to concerns with respect to the long-term safety of DES in this clinical setting [38]. On a clinical standpoint, meta-analyses of randomized trials have revealed an increased risk of very late ST with early-generation DES as compared with BMS in patients with acute MI [39,40]. The further development of DES has addressed this limitation. New-generation EES and BES have been compared with BMS in two largescale randomized trials [17,41]. In the EXAMINATION (everolimus-eluting stents versus bare-metal stents in ST-segment elevation myocardial infarction) trial [41], EES did not reduce the risk of the primary end point – a composite of all-cause death, any reinfarction and any revascularization – compared with BMS at 1 year (11.9 vs 14.2%; p =0.19). However, the risks of both target-lesion revascularization (2.1 vs 5.0%; p =0.003) and ST (0 vs 1.9%; p =0.019) were significantly lower with EES than BMS. In the COMFORTABLE (comparison of biolimus eluted from an erodible stent coating with bare metal stents in acute ST-elevation myocardial infarction) trial [17], BES with biodegradable polymer coating significantly reduced the risk of the primary end point – a composite of cardiac death, target-vessel reinfarction and ischemia-driven target-lesion revascularization at 1 year (4.3 vs 8.7%; p =0.004). The difference was driven by a lower risk of target-vessel reinfarction (0.5 vs 2.7%; p =0.01) and ischemia-driven target-lesion revascularization (1.6 vs 5.7%, p < 0.001) in patients receiving BES compared with those receiving BMS. ST was numerically less frequent with BES compared with BMS (0.9 vs 2.1%; p =0.10) without reaching statistical significance. A pooled analysis of the two trials, including a total of 2665 patients with acute MI, confirmed a marked improvement not only in terms of device effectiveness (target-lesion revascularization: hazard ratio [HR] 0.32, 95% CI: 0.20–0.52) but also in terms of device safety (definite ST: HR 0.35, 95% CI: 0.13–0.75; target-vessel reinfarction: HR 0.36, 95% CI: 0.14–0.91) with new-generation DES compared with BMS in patients with acute MI (Figure 4) [42]. In line with these data, the use of newgeneration DES in patients with acute MI is recommended over BMS (class I, level A) in the most recent guidelines on myocardial revascularization of the European Society of Cardiology (ESC) [43]. Therefore, the use of BMS in the setting of acute MI appears not to be supported by evidence or guidelines.
Figure 4. Clinical outcomes with new-generation drug-eluting stents versus bare-metal stents in patients with acute myocardial infarction.Device-oriented composite
end point (A) and its components cardiac death (B), target-vessel reinfarction (C) and clinically indicated target-lesion revascularization (D).
Adapted from [42].
Planned noncardiac surgery
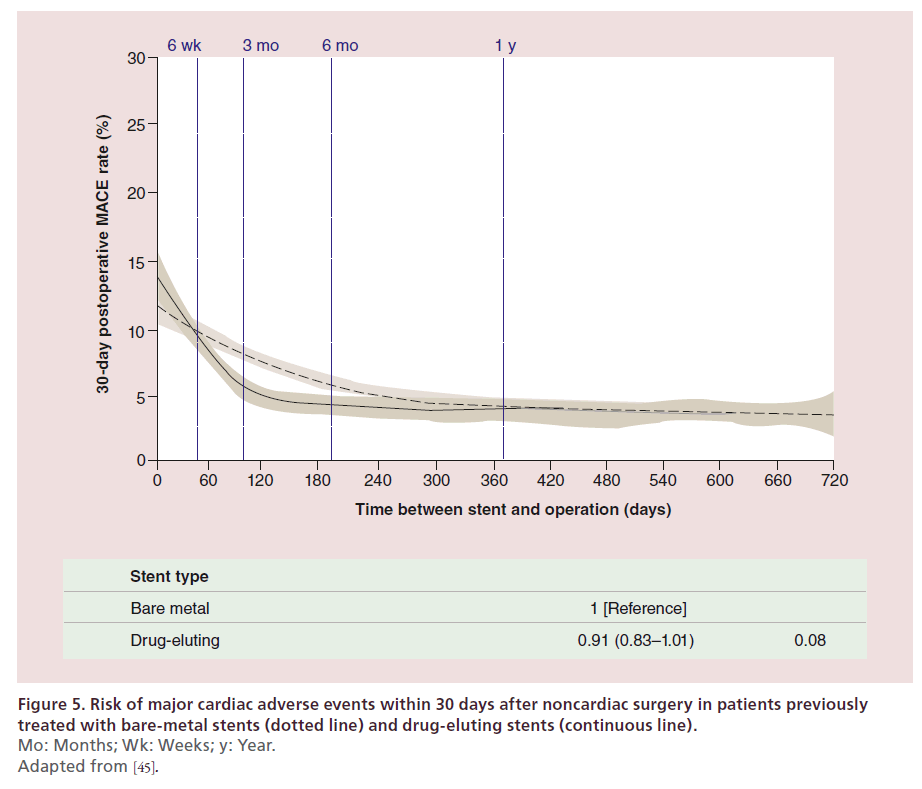
Noncardiac surgery is often needed in patients taking DAPT after PCI. Antiplatelet therapy in patients requiring surgical procedures is a matter of concern for surgeons due to the risk of bleeding events, and DAPT is frequently interrupted in patients undergoing noncardiac surgery. Cessation of DAPT prior to the recommended duration as well as the prothrombotic and proinflammatory state associated with surgery engender an increased risk of ischemic events in patients undergoing surgery recently after PCI with stent implantation. However, the risk of perioperative bleeding may be greater than the risk of ischemic events in such patients. BMS selection in these patients is a consequence of the concerns related to the potential premature DAPT interruption. In a retrospective cohort study, 8116 patients who underwent major elective noncardiac surgery between 2003 and 2009 and received stents within 10 years prior to surgery (i.e., between 1993 and 2009) were compared with a cohort of surgical patients who had not undergone myocardial revascularization [44]. Patients undergoing surgery <45 days after stenting had an increased risk of ischemic events irrespective of the type of stent implanted. The risk was reduced to the level of intermediate-risk nonrevascularized individuals >45 days after BMS implantation and >180 days after early-generation DES implantation. Of note, this study was limited to early-generation DES. Recently, a study has evaluated the risk of ischemic events according to previously implanted stent type in patients undergoing noncardiac surgery [45]. In this study, Hawn and colleagues retrospectively analyzed a cohort of 28,029 patients undergoing noncardiac surgery within 2 years after a coronary stent implantation (performed between 2000 and 2010) and examined the association between timing of surgery and stent type with major adverse cardiac events (MACE, defined as the composite of allcause mortality, MI and repeat revascularization). Time between stent implantation and surgery was associated with MACE (<6 weeks, 11.6%; 6 weeks to <6 months, 6.4%; 6–12 months, 4.2%; >12–24 months, 3.5%; p < 0.001). Moreover, emergency surgery (adjusted odds ratio [OR] 4.77; 95%CI: 4.07–5.59), history of MI in the 6 months preceding surgery (adjusted OR 2.63; 95% CI: 2.32–2.98), and revised cardiac risk index greater than 2 (adjusted OR 2.13; 95% CI: 1.85–2.44) were identified as independent predictors of MACE. Whereas, previous DES implantation was not associated with MACE (adjusted OR 0.91; 95% CI: 0.83–1.01) (Figure 5), suggesting no impact of stent type on the risk of ischemic events in patients undergoing noncardiac surgery after stent implantation. In the absence of prospective randomized studies, clinical practice should be guided by available observational evidence. The latter indicates an increased risk of ischemic events in patients undergoing surgery early after stent implantation (i.e., <6 weeks), irrespective of stent type. This risk appears to be reduced to normal in patients undergoing surgery >6 months after stent implantation, irrespective of stent type. Whether DES or BMS should be preferred in patients needing cardiac surgery between 6 weeks and 6 months after stent implantation is subject of ongoing debate.
Stent costs
A number of studies on cost–effectiveness suggested restricting the use of DES to patients at increased risk of restenosis in order to balance beneficial effects and costs [46–49]. Limiting the use of DES in patients at higher risk of restenosis may translate into cost savings with only a small impact on the risk of repeat revascularization procedures [46,47]. In the USA, a temporal reduction in DES use in 2007 (68% of patients receiving DES) as compared with a more liberal use in the years 2004–2006 (92% of patients receiving DES) resulted in a modest reduction in costs (400 USD per patient) and a small increase in the rates of repeat revascularization procedures (4.1 vs 5.1%) at 1 year [47]. Notwithstanding, recent analyses of US routine clinical practice indicated that the higher costs of DES and subsequent longer DAPT regimens are fully offset by a reduction in costs of repeat revascularization procedures compared with BMS at 3 years of follow-up [48]. It should be underscored that these analyses are based on early-generation DES with stent costs from year 2005 (i.e., >2100 USD), which are not used in current clinical practice. Indeed, a more recent study has observed a reduction of costs with EES compared with early-generation paclitaxeleluting stents, indicating that new-generation DES are economically more attractive [49]. Therefore, stent costs appear not to justify the use of BMS instead of DES at this point in time. It is noteworthy, however, that reimbursement systems between different countries and even within a given country, limit the generalizability of cost–effectiveness analyses.
Future perspective
As outlined in this article, currently available metallic DES have an excellent safety and efficacy profile, and represent the standard of care for PCI in current clinical practice as recommended by the most recent guidelines on myocardial revascularization of the ESC. Nevertheless, despite their impressive clinical results, the permanent presence of metallic DES represents a matter of concern in view of its lifelong compatibility with the implanted vessel. Fully bioresorbable vascular scaffolds (BVS) have been developed to overcome this limitation. These devices – made of polymers or magnesium alloys – offer scaffolding properties coupled with site-specific drug elution, followed by a complete resorption of the scaffold backbone. Preclinical studies and initial clinical investigations have provided evidence of complete bioresorption and some restoration of vascular physiology, without any safety concerns. However, limited evidence is available on the safety and efficacy profile of BVS as compared with contemporary metallic DES. Whether BVS will become the workhorse technology for PCI in the next few years will depend on the results of a number of ongoing randomized trials directly comparing them with metallic DES and on improvements in their ease of use which is not yet competitive.
Conclusion
A large body of evidence has shown a significant improvement in coronary stent safety and efficacy with device evolution. Early-generation DES have been superseded by new-generation DES, which optimized safety outcomes without compromising device effectiveness. The article, provides an overview of the most frequent reasons retained for BMS implantation. Reviewing the available evidence, there appears to be limited space left for the use of BMS in current clinical practice. Along this line, the most recent guidelines on myocardial revascularization of the ESC provide a class I level of evidence A recommendation for the use of new-generation DES over BMS in patients undergoing PCI irrespective of clinical presentation or antiplatelet regimen [43].
Executive summary
Why are we still using bare-metal stents?
• The most frequent reason is related to concerns on dual antiplatelet therapy (DAPT) compliance.
• Other reasons are the presence of a target vessel with a large reference diameter, treatment of patients with acute myocardial infarction, concerns related to reimbursement and planned noncardiac surgery within 1 year after stent implantation.
Concerns on dual antiplatelet therapy compliance
• Recent observational evidence suggests that the type of implanted stent (i.e., drug-eluting stents [DES] or bare-metal stents [BMS]) has no impact on the risk of ischemic events after DAPT cessation.
• Available evidence indicates that concerns on DAPT compliance should not prevent from the use of DES in patients undergoing percutaneous coronary interventions.
Large vessel diameter
• The superior antirestenotic effectiveness of DES over BMS was confirmed in large coronary arteries.
• The presence of a target vessel with a large reference diameter is not a valid reason for BMS implantation.
ST-segment elevation myocardial infarction
• New-generation everolimus-eluting stents and biolimus-eluting stents have been shown to improve outcomes compared with BMS in two large-scale randomized trials.
• The use of new-generation DES in patients with acute myocardial infarction is recommended over BMS (class I, level A) in the most recent guidelines on myocardial revascularization of the European Society of Cardiology.
Planned noncardiac surgery
• An increased risk of ischemic events in patients undergoing surgery early after stent implantation (i.e., <6 weeks) has been observed, irrespective of stent type (i.e., DES or BMS).
• This risk appears to be reduced to normal in patients undergoing surgery >6 months after stent implantation, irrespective of stent type (i.e., DES or BMS).
• Whether DES or BMS should be preferred in patients needing cardiac surgery between 6 weeks and 6 months after stent implantation is subject of ongoing debate.
Stent costs
• Available cost–effectiveness studies indicate that stent costs do not justify the use of BMS instead of DES.
• Of note, the generalizability of cost–effectiveness analyses is limited by the heterogeneity of reimbursement systems across different countries and even within a given country.
Future perspective
• Fully bioresorbable scaffolds offering scaffolding properties and controlled drug elution followed by complete resorption of the stent backbone have been developed.
• Whether fully biodegradable scaffolds will become the workhorse technology for percutaneous coronary interventions in the next few years will depend on the results of ongoing investigations.
Financial & competing interests disclosure
B Meier received research grants to the institution and speaker honoraria from Abbott Vascular, Biotronik, Biosensors, Boston Scientific, Johnson & Johnson and Medtronic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Stefanini GG, Holmes DR. Drug-eluting coronary-artery stents. N. Engl. J. Med. 368(3), 254–265 (2013).
- Serruys PW, Unger F, Sousa JE et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N. Engl. J. Med. 344(15), 1117–1124 (2001).
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346(23), 1773–1780 (2002).
- Grube E, Silber S, Hauptmann KE et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 107(1), 38–42 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Kastrati A, Mehilli J, Pache J et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 1030–1039 (2007).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591), 937–948 (2007).
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115(11), 1440–1455; discussion 55 (2007).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J. Am. Coll. Cardiol. 48(12), 2584–2591 (2006).
- Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 356(10), 1009–1019 (2007).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 989–997 (2007).
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356(10), 1020–1029 (2007).
- Kedhi E, Joesoef KS, McFadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 375(9710), 201–209 (2010).
- Stone GW, Rizvi A, Newman W et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl. J. Med. 362(18), 1663–1674 (2010).
- Stefanini GG, Kalesan B, Serruys PW et al. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. Lancet 378(9807), 1940–1948 (2011).
- Raber L, Kelbaek H, Ostoijc M et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA 308(8), 777–787 (2012).
- Bangalore S, Kumar S, Fusaro M et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 125(23), 2873–2891 (2012).
- Palmerini T, Biondi-Zoccai G, Della Riva D et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 379(9824), 1393–1402 (2012).
- Stefanini GG, Byrne RA, Serruys PW et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur. Heart J. 33(10), 1214–1222 (2012).
- Stefanini GG, Baber U, Windecker S et al. Safety and efficacy of drug-eluting stents in women: a patient-level pooled analysis of randomised trials. Lancet 382(9908), 1879–1888 (2013).
- Windecker S, Stortecky S, Stefanini GG et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ 348, g3859 (2014).
- Räber L, Magro M, Stefanini GG et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 125(9), 1110–1121 (2012).
- Tada T, Byrne RA, Simunovic I et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc. Interv. 6(12), 1267–1274 (2013).
- Sarno G, Lagerqvist B, Frobert O et al. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur. Heart J. 33(5), 606–613 (2012).
- Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127(1), e6–e245 (2013).
- Morice MC, Urban P, Greene S, Schuler G, Chevalier B. Why are we still using coronary bare-metal stents? J. Am. Coll. Cardiol. 61(10), 1122–1123 (2013).
- McFadden EP, Stabile E, Regar E et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 364(9444), 1519–1521 (2004).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Mehran R, Baber U, Steg PG et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 382(9906), 1714–1722 (2013).
- Silber S, Kirtane AJ, Belardi JA et al. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimus-eluting stent implantation. Eur. Heart J. 35(29), 1949–1956 (2014).
- Brunner-La Rocca HP, Kaiser C, Bernheim A et al. Cost-effectiveness of drug-eluting stents in patients at high or low risk of major cardiac events in the Basel Stent KostenEffektivitats Trial (BASKET): an 18-month analysis. Lancet 370(9598), 1552–1559 (2007).
- Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schomig A. Vessel size and long-term outcome after coronary stent placement. Circulation 98(18), 1875–1880 (1998).
- Kaiser C, Galatius S, Erne P et al. Drug-eluting versus bare-metal stents in large coronary arteries. N. Engl. J. Med. 363(24), 2310–2319 (2010).
- Kaiser C, Galatius S, Jeger R et al. Long-term efficacy and safety of biodegradable-polymer biolimus-eluting stents: main results of the Basel Stent Kosten-Effektivitats Trial-PROspective Validation Examination II (BASKET-PROVE II), a randomized, controlled noninferiority 2-year outcome trial. Circulation 131(1), 74–81 (2015).
- Spaulding C, Henry P, Teiger E et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N. Engl. J. Med. 355(11), 1093–1104 (2006).
- Stone GW, Lansky AJ, Pocock SJ et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N. Engl. J. Med. 360(19), 1946–1959 (2009).
- Nakazawa G, Finn AV, Joner M et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 118(11), 1138–1145 (2008).
- Kalesan B, Pilgrim T, Heinimann K et al. Comparison of drug-eluting stents with bare metal stents in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 33(8), 977–987 (2012).
- De Luca G, Dirksen MT, Spaulding C et al. Drug-eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials. Arch. Intern. Med. 172(8), 611–621; discussion 21–22 (2012).
- Sabate M, Cequier A, Iniguez A et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 380(9852), 1482–1490 (2012).
- Sabate M, Raber L, Heg D et al. Comparison of newer-generation drug-eluting with bare-metal stents in patients with acute ST-segment elevation myocardial infarction: a pooled analysis of the EXAMINATION (clinical Evaluation of the Xience-V stent in Acute Myocardial INfArcTION) and COMFORTABLE-AMI (Comparison of Biolimus Eluted From an Erodible Stent Coating With Bare Metal Stents in Acute ST-Elevation Myocardial Infarction) trials. JACC Cardiovasc. Interv. 7(1), 55–63 (2014).
- Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 35(37), 2541–2619 (2014).
- Wijeysundera DN, Wijeysundera HC, Yun L et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 126(11), 1355–1362 (2012).
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 310(14), 1462–1472 (2013).
- Amin AP, Spertus JA, Cohen DJ et al. Use of drug-eluting stents as a function of predicted benefit: clinical and economic implications of current practice. Arch. Intern. Med. 172(15), 1145–1152 (2012).
- Venkitachalam L, Lei Y, Stolker JM et al. Clinical and economic outcomes of liberal versus selective drug-eluting stent use: insights from temporal analysis of the multicenter Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) registry. Circulation 124(9), 1028–1037 (2011).
- Schafer PE, Sacrinty MT, Cohen DJ et al. Cost-effectiveness of drug-eluting stents versus bare metal stents in clinical practice. Circ. Cardiovasc. Qual. Outcomes 4(4), 408–415 (2011).
- Amin AP, Reynolds MR, Lei Y et al. Cost-effectiveness of everolimus- versus paclitaxel-eluting stents for patients undergoing percutaneous coronary revascularization (from the SPIRIT-IV Trial). Am. J. Cardiol. 110(6), 765–770 (2012).