Research Article - Clinical Investigation (2023) Volume 13, Issue 1
A new generation of multitarget and safe topical pharyngitis treatment for children: Clinical efficacy vs Saline solution
- Corresponding Author:
- Rémi Shrivastava
Research Scientist in Neurology, PhD, University of Clermont Auvergne, Department of Neurology- Neuro-Dol, BP 10448, F-63000, Clermont-Fd, France
E-mail: remi.s@naturveda.fr
Abstract
Background: Pharyngitis is the most common disease in children involving viral infection, throat mucosa cellular damage, secondary bacterial infection, inflammation, and broken cellular mucosa integrity. When clinical signs appear, the disease has already become multifactorial where only a multi-target treatment can provide quick relief. In the absence of any multi-target treatment, we conceived a new generation of topical, osmotic, throat surface cleaning polymeric film, capable of detaching and draining throat surface contaminants nearly instantly. The clinical efficacy and safety of this medical device is evaluated in children.
Methods: The test product was conceived by rendering osmotic glycerol filmogen and stable through specific polymeric binding. An observational, randomized, placebo-controlled study was performed after the approval of ethics committee on 30 children aged between 3 to 15, presenting symptoms of acute pharyngitis. After randomization, test product (n=20) and saline control (n=10) solutions were applied as 3 spray to 4 spray on the throat, 2 times to 3 times per day for 15 days. Change in all the key pharyngitis symptoms (difficulty swallowing, throat inflammation, irritation, redness, bacterial deposit, need for antibiotics) were evaluated.
Results: The test product was highly efficient in significantly supressing all the pharyngitis symptoms vs comparator product within 3 days of treatment. The need for antibiotics was drastically decreased and no adverse effects were noticed during the study.
Conclusions: Detaching and draining all the free-floating throat surface contaminants with a nearly instant, polymeric osmotic film, without the use of any chemical drug, represents a totally new approach for the treatment of pharyngitis in children. This mechanically acting, multi-target, new generation of polymeric drugs can help reduce the problem of antibiotic resistance and long-term side effects of currently used pharyngitis drugs in children.
Introduction
Acute pharyngitis is one of the most common illnesses for which children visit primary care physicians. Clinical manifestations include sore throat and fever with sudden onset, red inflamed throat, enlarged tonsils covered with yellowish or whitish deposits, blood-tinged exudate on the soft palate and throat area, swollen anterior cervicalnodes, throat pain, fever, fatigue, headache, and occasional gastrointestinal symptoms [1].
Secondary signs may also include runny nose, cough, itchy and watery eyes. In 80% of cases, the origin of the disease is viral (rhinovirus, adenovirus, parainfluenza virus, coxsackievirus, coronavirus, cytomegalovirus, mononucleosis) but can also be secondary to environmental irritants [2, 3]. Initially, virus attacks a few throat mucosa cells, starts growing, causes cellular damage, and when the cells die, hundreds of new free viral particles are released on the throat surface [4, 5]. The disease is nearly asymptomatic up to this stage. These newly liberated virus particles now attack new healthy cells, cause widespread tissue damage, open gaps on the intact mucosa barrier and trigger a strong immune reaction. Tissue damage creates a favorable environment for the growth of opportunistic bacteria, particularly Group A Streptococcus (GAS), releasing Streptococcal Pyrogenic Exotoxins (SpeA, SpeC, SpeG to SpeM), Superantigen A (SSA), and Mitogenic Exotoxin-Z (SmeZ) [2]. They activate a large proportion of T-cell population, of which innate immune cells (monocytes, macrophages, dendritic cells, T cells, NK lymphocytes…) elicit an excessive and uncoordinated release of pro inflammatory cytokines such as TNF-α, IL-6, IL-10, and IL-12 on the throat surface, causing throat inflammation and pain [6, 7]. When symptoms start appearing, the disease already became multifactorial involving viral growth, extensive throat mucosa damage, gaps on the throat mucosa allowing systemic entry of contaminants, abundant pro inflammatory cytokines and growing microorganisms on the throat surface, visible as whitish deposits on the throat surface [8, 9]. Symptoms appear only when the disease has largely progressed, microorganisms are installed, and inflammation has started to damage throat surface cells. The disease physiopathology shows that pharyngitis is a multifactorial disease which will require multiple treatments, each directed to counteract a specific factor, to cure the disease rapidly. One may argue that the natural defense of the body should help irradiate the pathogens and repair the damaged tissue, but children’s natural defenses are not sufficiently mature to suppress the pathogens rapidly, which delays healing and increases the chances of disseminating the infection in other organs of the body. Children being more fragile, suffer more than adults and need 3 week to 4 week for natural recovery [10].
Treating such a multifactorial disease compulsorily requires a multi-target treatment but in the absence of any such drug, all the current pharyngitis treatments are single target oriented chemical drugssuch as analgesics, antipyretics, antibiotics, anti-inflammatory, and anesthetics, which constitute the first-line symptomatic remedies [11]. Being mono-target treatments; physicians usually associate different drugs and add antibiotics to prevent further infection [12, 13]. There is no treatment to clean and to repair damaged throat mucosa, an essential prerequisite to avoid further progression of the disease.
Taking into consideration these multiple yet essential properties for an efficient pharyngitis treatment, only a treatment which can physically clean the throat surface may achieve these objectives. Among the available remedies, except for saline or saltwater gargling, there is nothing else which can possess these basic requirements [14]. Saline or salt solutions containing up to 3.2% salt act physically and osmotically to clean the throat, but their efficacy is low because they are not very osmotic, not stable, short acting, and require applications every 3 h to 4 h for 2 days to 3 days, to obtain satisfactory results [15, 16].
Therefore, to be effective, the basic treatment strategy should be multi-target, directed at rapidly cleaning the throat surface; detaching and removing microbial deposits; inflammatory proteins, and other contaminants from the throat surface to provide ideal conditions for the repair of damaged throat mucosa. In addition, for children, such a multitarget treatment should also be free of side effects, nonchemical and non-irritant, and easily applicable.
To foresee such a multi-target treatment for children, we envisaged conceiving a glycerol-based, absorbent, osmotic, non-irritant, and film-forming solution, which can be rendered mechanically resistant by incorporating glycerol-molecule-binding natural polymer [16, 17]. It was postulated that topical application of such a solution on the throat surface as a film, should generate a strong osmotic liquid flow from the throat tissues, to detach and drain surface contaminants towards the film where they may be absorbed. Removing bacteria and freefloating protein molecules should contribute to reducing pain, inflammation, cough-reflex, and in consequence should provide a favorable environment for throat mucosa cell growth to grow and repair the damage. As this mechanically acting film is prepared from natural and non-cytotoxic ingredients, it should not have undesired side-effects or cause problems of microbial resistance.
The clinical efficacy and safety of this natural polymeric throat film for the treatment of pharyngitis in children is evaluated compared to saline solution.
Methods
Clinical trial oversight
The study was designed as a randomized, doubleblind, placebo-controlled study to evaluate efficacy and safety of the Test Product (TP) compared to 0.9% saline solution sprays as Comparator Product (CP). The trial (Protocol: VB-PHR/OBS/2018) was conducted by Mudra CLINCARE, located at Awaskar Building 402107 Mumbai, India, certified to conduct clinical investigations on human subjects (N°UQ-2022122821 following ISO-14155 guidelines). The study was coordinated by Dr. Sayali and was performed at Malsons Multispeciality Hospital and ICU, Navi Mumbai, MS, India, as per the regulations applicable for studies in children. The protocol was approved by relevant ethics committees (Altezza Institutional Ethics Committee, Shree Ashirwad Hospital, Dombivli, Maharashtra, India and institutional review boards (Reg. No. ECR/247/Inst/MH/2013/RR-16, dated 07/08/2018). The authors vouch for the conduct of the trial, adherence to the protocol, the accuracy and completeness of the data, and reporting of adverse events. The trial complied with the International Conference on Harmonization Guidelines for Good Clinical Practice, the principles of the Declaration of Helsinki, and relevant national and local regulations. At the time of screening, the children’s parent(s) signed written informed consent forms. The sponsor provided the trial medication (ISO 13485 certified) and supplied relevant investigation product information.
Test and comparator products
The Test Product (TP), designated as Throat-Kid spray, commercialized in Europe as a medical device, was supplied by Vitrobio Pharma in France (ISO13485 certified) in 20-ml aluminium containers fitted with a spray for throat application. The solution was prepared by combining osmotically active filmogen glycerol as described by Shrivastava et al [18]. which was rendered filmogen by adding small quantities of glycocyanidin-e polymericpremix derived from Vitis vinifera seeds, Sambucus nigra and Ribes nigrum fruit; honey as a thickener, qsp water. The CP was presented identically to the TP and contained 0.9% NaCl solution. TP and CP were applied as 3 spray to 4 spray on the throat surface every 20 min to 30 min during the first 2 h at the start of treatment and 2 time to 3 time per day up to day 15 or till complete recovery
Trial participants
Based on previous studies of similar products using the same technology and in order to achieve a minimum statistical power of 80%, it was recommended to include a minimum of 30 patients divided in a 2:1 ratio between the treatment group and the placebo group [19].
The 1st patient was recruited on 18th September 2018, the last treatment was completed on 27th October 2018, and results were reported on 11th January 2019.
Key inclusion criteria
• Boys and girls aged 3 to 18
• Having symptoms of recent throat infection (<72 h), temperature>38°C, swelling of tonsils with >50% probability of positive culture for Streptococcus infection.
• Parents ready to provide written informed consent.
• Ready to abstain from other treatments except antibiotics, in seriously ill patients to evaluate requirements of anti-biotherapy.
• Ready to follow the protocol and fill-in the daily questionnaire diary.
• Not under any antiviral or anti-inflammatory treatment.
• Not suffering from any chronic disease which may impact study parameters.
The main exclusion criteria
• Not meeting any of the above inclusion criteria.
• Allergy to any of the investigational product ingredient.
• Any disease or treatment which may affect study outcome.
• Parents' refusal to submit to the protocol.
Randomization
Treatments were allocated to patients by carrying out randomization using SAS Version 9.1.3. Biostatistician generated the randomization schedule.Block Randomization methodology was employed to follow 2:1 ratio in TP or CP groups.
Trial endpoints
Pharyngitis and/or throat infection related parameters were scored on a 1 to 7 scale (1=excellent; 7=worst). Key parameters recorded were difficulty in swallowing, swollen throat, throat inflammation/redness, throat irritation, and presence of whitish microbial deposit on the throat surface. Throat swabs were examined at the start, Baseline (BL) and at the end of the study (Day 15). Number of days of antibiotic use (if any), in each group was also recorded. Mean scores of each symptom, at each time point, were compared with the baseline (BL: start of treatment) and between the groups on day 1 (2 h after first treatment), day 3, day 7 and day 15 (end of the study).
Statistical analyses
Analyses were conducted in the modified intentionto-treat population, which included all randomly assigned patients who received at least one dose of a trial. Safety analyses included all randomly assigned patients who received at least one dose of a trial product. Demographic and baseline characteristics were summarized descriptively with a student’s test for comparison between the two groups and Fisher’s exact test for analysis of contingencies. The primary efficacy outcome was analysed with two-way repeated measure ANOVA followed by the post hoc Bonferroni’s test. The mean change from the baseline with SEM is presented for each treatment group, and the difference versus placebo with 95% Confidence Interval (CI). The same analyses were used for relevant secondary end points. P<0.05 was considered statistically significant. Statistical analysis was performed by Chi-square test for comparison of adverse events between the two groups. The analyses were carried out with the software GraphPad Prism 9.5 (La Jolla, USA).
Results
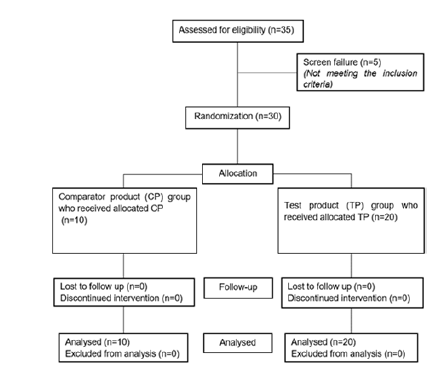
DemographicsAs shown in the Consort chart , a total of 35 patients were recruited, 5 did not meet inclusion criteria, and 30 were randomized in 2 groups (Figure 1). 20 patients (7 boys and 13 girls) aged between 4 year to 14 year (mean 8.3 ± 2.87) were included in the TP group while 10 patients, aged between 3 year to 13 year (mean 7.9 ± 3.98) were allocated in CP group.
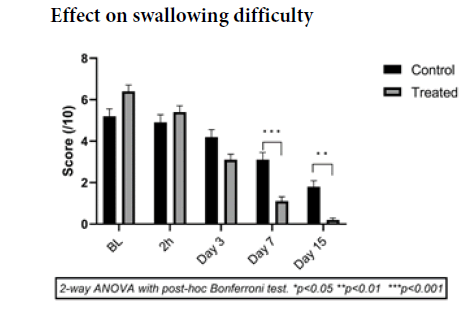
At baseline the scores did not differ significantly between the two groups (p=0.097) with a mean of 5.2 ± 0.5 for the control group and 6.4 ± 0.5 for the treatment group (Figure 2).
From the third day, a trend starts to show a difference in evolution between the two groups with a p value close to significance: difference of 1.1 (-0.18 to 2.39) p=0.12 between the two groups. The control group showed a 19% decrease from baseline compared to 52% in the treatment group.
At day 7 the difference between the two groups was 2.0 (0.80 to 3.19) p<0.001, which confirms the trend observed from day 3.
The observed results show the effectiveness of the tested treatment to improve swallowing in children with throat infections.
The results observed for swelling corroborate those for swallowing. From the 3rd day, the difference between the two groups is 1.05 (-0.03 to 2.13) p=0.058 that is to say a p-value very close to the significance (Figure 3). This trend continued and became significant on day 7 with a difference between the two groups of 1.3 (0.26 to 2.34) p=0.011. Throat swelling was decreased in both groups, but the reduction was much stronger in the TP group. Swollen throat symptom was still present in CP group on day 15 but all the patients in the TP group had totally recovered. The osmotic liquid exudation generated by the TP film should have contributed in reduction of swelling.
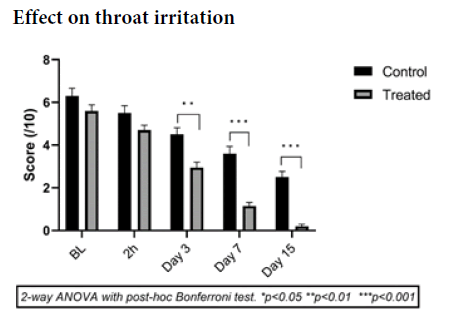
Effect on throat irritation
The effect on throat irritation was observed from the second hour of treatment with a difference between the two groups of 0.8 (-0.39 to 1.99) p=0.34 in favor of the treated group. This trend was confirmed and became significant on the third day with a difference between the two groups of 1.55 (0.42 to 2.68), i.e. an improvement of 29% in the placebo group compared to 47% in the treated group.
This difference increased until the end of the treatment with a p-value for the difference between the two groups lower than 0.001 from the seventh day in favor of the treated group (Figure 4).
These results show the ability of the treatment to rapidly and significantly reduce throat irritation in children.
Effect on throat redness (inflammation)
The redness of the throat, which is a reflection of its inflammation, follows the same pattern as the previous results. Because inflammation is a long process to disappear, no trend is observed before the 3rd day (p>0.99), which is normal in view of the previous results (Figure 5). On the other hand, a significant difference in favor of the treated group appeared as early as day 3: 1.5 (0.49 to 2.51) p=0.002, i.e., a decrease of 28% in the placebo group compared to 56% in the treated group.
On the following days, the results show that inflammation continues to decrease with a difference of 2.15 (1.15 to 3.15) p<0.001 on day 7.
Figure 5: Mean scores of throat inflammation (MEAN ± SEM) recorded by physicians in the treated (n=20, gray bars) vs control (black bars, n=10) groups on a 0-10 scale (0=no inflammation)
Strong reduction of inflammation in TP group may have been related to the mechanical cleaning and removal of inflammatory proteins from the throat surface due to osmotic liquid exudation.
Effect on whitish deposit on throat
On day 1, there was no significant change in mean scores for the presence of whitish bacterial deposits on the throat surface compared to BL, in both groups (Figure 6). From day 3, the results show a sharp decrease in whitish / yellowish throat surface deposits in TP group (-56.38%, p<0.01 vs CP from day 3 onwards) with further reduction on day 7 (-80.85%) and day 15 (-97.87%). In CP group, bacterial growth also reduced with time indicating a mean decrease of 22.22% on day-3, 46.29% on day-7, and 70.37% on day 15. Results indicate that although there is a progressive reduction of bacterial growth with time, taking about 15 days to 20 days to disappear in the absence of treatment, microbial growth reduces faster when the throat surface is cleaned every 3 h to 4 h with saline solution. The same results are obtained with the TP but this reduction is very fast and strong vs CP. The TP, being an osmotic solution, strongly attracts hypotonic liquid which helps detachment of strongly adhered microorganisms from the throat surface.
Figure 6: Visual scoring of whitish deposits (bacterial growth) by physicians in the treated (n=20, gray bars) vs control (black bars, n=10) groups on a 0-10 scale (0=not observed). Both saline solution and test product help in reducing bacterial deposits during the entire treatment period compared to BL values, but this reduction is much faster in the TP group. The TP vs CP throat surface bacterial growth difference is statistically significant from day 3 onwards (p<0.01 vs CP from day 3 onwards)
Throat swab analyses: At the start of the study, 9/10 (90%) children in the CP group and 16/20 (80%) in the TP group had a positive throat swab for the presence of bacteria. At the end of the study, only 2/10 (20%) children in the CP group and 3/20 (15%) in the TP group were positive, showing that bacterial contamination on the throat had sharply decreased in both groups but this decrease was stronger in the TP group.
Use of antibiotics: In the CP saline treated group, 40% children (4/10) required antibiotic therapy for a mean period of 6.3 days compared to only 15% (3/20) in the TP group for a mean period of 5.3 days.
Adverse events: No related adverse events were recorded in either group throughout the study.
Overall product assessment: Patients and investigators were asked to rate treatments’ efficacy as excellent, very good, good, or fair for both products. The corresponding scores for CP and TP groups were 0%, 10%, 65%, 25% for CP and 72%, 25%, 2.5% and 0% for TP group indicating that the test product efficacy and safety were highly appreciated by the users.
Discussion
Pharyngitis is one of the most common upper respiratory tract infections in children and the incidence is nearly 3-times more common in children then in adults [20]. Infection transmission rate is nearly 50% [21]. Even though the origin of pharyngitis in children is mostly viral, secondary bacterial infection is the main cause of disease related symptoms, tissue destruction, and secondary complications. Group A - hemolytic Streptococcus (GAS) is considered the most pathogenic microorganism in children and accounts for 20% to 30% sore throat medical visits [10, 22]. If left untreated, it may cause post streptococcal glomerulonephritis and Acute Rheumatic Fever (ARF) that is currently uncommon in most developed countries but remains the leading cause of acquired heart disease and rheumatic mitral stenosis in developing and low income group countries [23]. Antibiotics and anti-inflammatory drugs always constitute first line of treatments but due to increasing microbial resistance to antibiotics, GAS is becoming a major health threat to children, even in developed countries [5, 24].
If the cause of the disease is not suppressed within 3 initial days-5 initial days, multiple pro inflammatory cytokines continue being released and accumulate on the throat surface, inflicting inflammation, and creating cellular gaps through which infectious organisms and cytokines can easily enter systemic circulation. Significantly higher blood levels of inflammatory cytokine (TNF-α>1 pg/ml, IL-6>7 pg/ml) were detected in most of pharyngitis patients as opposed to healthy controls. Clinical parameters of infection are also significantly correlated with high levels of circulating inflammatory cytokines [25].
In children, infected throat mucosa represents a complex physio-pathological chain of events only because, initially, a pathogen has killed a cell and caused initial cellular damage! The throat surface contains growing viruses, bacteria, microbial degradation products, cellular debris, dead cells, multiple pro inflammatory and antiinflammatory cytokines and other proteins on the surface. These substances continue damaging URT mucosa, causing pain, irritation, itching, inflammation, systemic damage, and poor quality of life [9, 10]. Therefore, an effective treatment should target all these factors simultaneously. The aim should be to remove contaminants, reduce inflammation, repair damaged mucosa, stop further infection, and reconstitute natural URT mucosa barrier to stop the disease process as early as possible. A good treatment should also be safe, nonirritant and should preferably act exclusively on the throat surface, where these physio pathological events are occurring
Unfortunately, almost all currently available and commonly used treatments are mono-target, symptomatic, and contain chemicals which are not cell friendly. Pain killers and NSAIDs such as paracetamol and ibuprofen help to reduce pain and inflammation; anesthetics such as topical lidocaine only reduce pain sensation; antibiotics like penicillin or amoxicillin minimize bacterial contamination, but these drugs are not totally safe, especially if given too frequently to children. Paracetamol ingestion in very young children increases the probability of hepatotoxicity ibuprofens can damage gastrointestinal system epithelium, induce vomiting and in extreme cases kidney damage corticosteroid therapy may lead to increased appetite, weight gain, fluid retention, and gastrointestinal disorders while the use of antibiotics is not always justified or appropriate and may lead to long-term bacterial resistance, which has already become a global health threat [26-29]. Except in developed countries, the use of antibiotic treatment for pharyngitis is very common with increasing incidences of antibiotic resistant bacteria that are responsible for high rate of neonate mortality in Southeast Asian countries and over 99,000 deaths in the US. Above 700,000 deaths per year world wide are estimated to be due to antimicrobial resistance and these figures are increasing each year [30-33].
Therefore, our aim was to simply clean the throat surface and keep it clean up to the time the throat mucosa is not reconstituted. Cells can grow and repair the damages very rapidly, within 48 h, if their environment is clean, free from growing bacteria, cytotoxic chemicals, inflammatory substances, and other cell growth hindering contaminants. We achieved these manifold objectives using a topically applicable, cell-friendly, non-irritant, viscous natural ingredient: glycerol. Glycerol is nearly 18 times more osmotic than sea water and capable of generating strong osmosis when applied on a semi-permeable live biological surface [16]. Unfortunately, glycerol itself gets diluted within a few seconds through the hypotonic liquid it attracts from the cells. We rendered glycerol filmogen by binding and stabilizing glycerol molecules with specific natural polymeric molecules having affinity for glycerol [17]. Polymers are very big, inert, non-cytotoxic, and safe for topical application. Polymer-bound glycerol conserves its osmotic properties and forms a film which resists mechanical pressure for up to 4 h-6 h and ensures a longlasting live biological membrane mechanical cleaning property [18].
The throat mucosa biological cell membranes are semipermeable and regulate the exchange of fluid and nutrients through passive and active transport. Passive transport mechanisms drive water or solutes to move down their concentration gradients without need of energy input, whereas active transport mechanisms propel solutes to move against their concentration gradients at the cost of energy input from metabolic reactions [34]. The presence of a glycerol osmotic film on the throat surface with much higher osmolarity compared to intracellular liquid, separated by the semipermeable cell membrane, generates passive exudation of intracellular hypotonic fluid from the inside towards the outside of the throat mucosa cell. This continuous and strong liquid flow helps to detach and drain all the free-floating throat surface contaminants towards the TP film. The lost intracellular liquid from the throat cells is immediately replaced by circulating fluid without any effect on cellular physiological functions
In this study, the osmotic glycerol film was applied directly on the throat surface for 15 consecutive days but most of the key symptoms of pharyngitis were reduced by about 50% just within 3 days and by 80% to 99% between 10 days to 15 days. Just 72 h after the start of application, in the TP group, mean swallowing difficulty symptoms were decreased by 51% (32% in CP), throat swelling by 53% (vs 16% in CP), throat irritation by 47% (vs 32% in CP), and throat inflammation by 56% (vs 27% in CP) with a high recovery rate within a week. These results show that the TP is much more effective compared to CP in rapidly reducing clinical symptoms of pharyngitis in children.
The rapid decrease in the presence of whitish bacterial colonies on the throat surface with TP were due to osmotic mechanical detachment of bacteria which are then either trapped in the film or ingested in the stomach. Detached and digested bacteria cannot grow which prevented further infection and consequently reduced the need for antibiotic treatment. During this study, only 15% children in the TP group were given antibiotics vs 40% in the CP group. Such mechanically acting antimicrobial approaches can help minimize the use of antibiotics for most topical, oral, URT, genital, and skin infections where they can be removed mechanically. The absence of systemic or local side effects also proves the exclusively topical mode of action of TP with no absorption in the body and no undesired metabolic or immunological disturbances.
Children are fragile and continuous administration of any chemical drug for a week or more, may lead to long-term side effects, antibiotic resistance, drug interactions and increased risk of developing nephropathy, atopic dermatitis, asthma, allergic reactions, and weight gain [35]. Therefore, just spraying an osmotic yet non-irritant and stable solution on children’s throat surface to get rid of pharyngitis within 2 days to 3 days represents an important breakthrough in the treatment of pediatric pharyngitis.
Conclusion
Pharyngitis is a very common infection in children involving viruses, bacteria, cellular damage, and inflammation which cause severe throat pain, inflammation, and swelling. The disease progression should be stopped rapidly to avoid secondary infection. Being a multi-factorial disease, usually multiple chemical treatments are administered simultaneously, which assist in reducing the disease duration and provide symptomatic relief, but they are slow-acting and may have side-effects. We developed a totally new and multi-target, filmogen, osmotic throat cleaning device, which acts nearly instantly as a mechanical antibacterial, anti-inflammatory, analgesic, and totally safe device. This treatment is now available in many countries and is registered as a medical device.
Funding
This research received no external funding and was entirely financed by VITROBIO Pharma Research Institute in France.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors sincerely thank Mr. Thierry Guillemaut, Technical Director of Biocorp France for preparing clinical trial samplesReferences
- Arnold JC and NizetV. Pharyngitis. Princ Pract Pediatr Infect Dis. 199–205(2018). [Google Scholar] [Crossref]
- Sykes EA, Wu V, Beyea MM et al. Pharyngitis: Approach to diagnosis and treatment. Can Fam Physician. 66(4):251-57(2020). [Google Scholar] [Crossref]
- Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat). Inflamm Res. 61(10):1041-52(2012). [Google Scholar] [Crossref]
- Viral Infections Of The Oral Mucosa, in StatPearls. StatPearls Publ Treasure Isl. (2022) [Google Scholar] [Crossref]
- Ivaska L, Putkuri N, Vuopio J et al. Detection of group A streptococcus in children with confirmed viral pharyngitis and antiviral host response. Eur J Pediatr. 181(12):4059-65(2022)Google Scholar]
[Crossref]
- Geißler K, Weigel C, Rubio I, et al. Cytokine production in patients with recurrent acute tonsillitis: analysis of tonsil samples and blood. Sci Rep. 10(1):13006(2020) Google Scholar] [Crossref]
- Dinis M, Plainvert C, Kovarik P, et al. The innate immune response elicited by Group A Streptococcus is highly variable among clinical isolates and correlates with the emm type. PLoS One. 9(7):101464(2014) Google Scholar]
[Crossref]
- Flood S and Woods J. Academic Life of Emergency Medicine. Strep Pharyngitis in Children: Review of the 2012 IDSA Guidelines. (2019) Google Scholar] [Crossref]
- Schmutzler L, Mirna M, Hoppe U et al. From Streptococcal Pharyngitis/Tonsillitis to Myocarditis: A Systematic Review. J Cardiovasc Dev Dis. 9(6)(2022) Google Scholar]
[Crossref]
- Miller KM, Carapetis J, Beneden C et al. The global burden of sore throat and group A Streptococcus pharyngitis: A systematic review and meta-analysis. EClinicalMedicine. 48:101458(2022) Google Scholar]
[Crossref]
- Bouroubi A, Donazzolo Y, Donath F, et al. Pain relief of sore throat with a new anti-inflammatory throat lozenge, ibuprofen 25 mg: A randomised, double-blind, placebo-controlled, international phase III study. Int J Clin Pract. 71(9):(2017) Google Scholar]
[Crossref]
- Palm J, Fuchs K, Stammer H, et al. Efficacy and safety of a triple active sore throat lozenge in the treatment of patients with acute pharyngitis: Results of a multi-centre, randomised, placebo-controlled, double-blind, parallel-group trial (DoriPha). Int J Clin Pract. 72(12):13272(2018) Google Scholar]
[Crossref]
- Norton L and Myers A. The treatment of streptococcal tonsillitis/pharyngitis in young children. World J Otorhinolaryngol Head Neck Surg. 7(3):161-65(2021)Google Scholar]
[Crossref]
- King D, Mitchell B, Williams C, et al. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Re. (4):Cd006821(2015)
- Shrivastava R, Deshmukh S, Rousse M, et al. Comparison of a Hypertonic Tannin-rich Solution vs 3% NaCl Solution as Treatment for Rhinosinusitis. Am J Pharmtech Res.3(2)(2013) Google Scholar]
[Crossref]
- Shrivastava R. Non-solid composition for local application. (2000).
- Sykes EA, Wu V, Beyea MM et al.
- Shrivastava R, Shrivastava L. Dual acting polymers in an osmotic film for topical application to treat inflammatory diseases and cytokine release syndrome. (2022).
[Google Scholar] [Crossref]
- Rousse M, Schütte H, Guy M, et al. A randomized, double-blind, controlled study to evaluate clinical efficacy and safety of novel filmogen osmotic treatment for pharyngitis. Clin Investig. 7(2):75-88(2017). [ Google Scholar] [Crossref]
- Vukmir RB. Adult and pediatric pharyngitis: a review. J Emerg Med. 10(5):607-16(1992) Google Scholar]
[Crossref]
- Robinson JL. Paediatrics: how to manage pharyngitis in an era of increasing antimicrobial resistance. Drugs Context. 10(2021) Google Scholar]
[Crossref]
- Pearce S, Bowen AC, Engel ME, et al. The incidence of sore throat and group A streptococcal pharyngitis in children at high risk of developing acute rheumatic fever: A systematic review and meta-analysis. PLoS One. 15(11):0242107(2020) Google Scholar]
[Crossref]
- Karevold G, Kvestad E, Nafstad P, et al. Respiratory infections in schoolchildren: co-morbidity and risk factors. Arch Dis Child. 91(5):391-5(2006) Google Scholar]
[Crossref]
- Duong QA, Pittet L, Curtis N, et al. Antibiotic exposure and adverse long-term health outcomes in children: A systematic review and meta-analysis. J Infect. 85(3):213-300(2022).
- Anderson J, Imran S, Frost H, et al. Immune signature of acute pharyngitis in a Streptococcus pyogenes human challenge trial. Nat Commun. 13(1):769(2022) Google Scholar] [Crossref]
- Anderson BJ. What we don't know about paracetamol in children. Paediatr Anaesth. 8(6):451-60(1998) Google Scholar]
[Crossref]
- De MM, Chiarugi A, Montini G, et al. Working Towards an Appropriate Use of Ibuprofen in Children: An Evidence-Based Appraisal. Drugs. 77(12):1295-311(2017) Google Scholar] [Crossref]
- Deshmukh CT. Minimizing side effects of systemic corticosteroids in children. Indian J Dermatol Venereol Leprol. 73(4):218-21(2007) Google Scholar] [Crossref]
- Eliopoulos GM, Cosgrove SE, Carmeli Y. The Impact of Antimicrobial Resistance on Health and Economic Outcomes. Clin Infect Dis. 36(11):1433-37(2003) Google Scholar]
[Crossref]
- Alumran A, Hurst C, Hou XY. Antibiotics Overuse in Children with Upper Respiratory Tract Infections in Saudi Arabia: Risk Factors and Potential Interventions. Clin Med Diagn. 1(1):8-16(2011)
- Peters L, Olson L, Linnros S, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: A cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS One. 14(5):0215666(2019) Google Scholar]
[Crossref]
- Agarwal, Ramesh, Sankar. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health. 4(10):752-60(2016). [ Google Scholar] [Crossref]
- Klevens RM, Edwards J, Richards C, et al. Estimating health care-associated infections and deaths in U.S. hospital. Public Health Rep. 122(2):160-6(2002) Google Scholar] [Crossref]
- Chen I Lui F. Physiology, Active Transport, in StatPearls. StatPearls Publ Treasure Isl. (2022) Google Scholar] [Crossref]
- Gong J, Ma L, Li M, et al. Nonsteroidal anti-inflammatory drugs associated acute kidney injury in hospitalized children: A systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 31(2):117-27(2022).