Case Report - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 4
A challenging differential diagnosis: Granulomatosis with polyangiitis and tuberculosis
- Corresponding Author:
- Jaime Velásquez Franco
4Rheumatology Department
School of Health Sciences
Universidad Pontificia Bolivariana
Clinica Universitaria Bolivariana
Medellin, Colombia
E-mail: carjaivel@gmail.com
Abstract
This article reviews the case of a patient with a headache, palpable purpura, chronic otomastoiditis, renal involvement, and pulmonary granulomatous disease, with the concurrence of granulomatosis with polyangiitis and tuberculosis. The association between both diseases and the challenge in the differential diagnosis are discussed.
Keywords
Granulomatosis with polyangiitis • anti-neutrophil cytoplasmic antibody-associated vasculitis • tuberculosis • fever • otitis media
Introduction
Granulomatosis with polyangiitis (GPA) is a medium and small-vessel vasculitis associated with positive anti-neutrophil cytoplasmic antibodies (ANCA), which generate a necrotizing granulomatous inflammation, mainly in the upper and lower respiratory tract, up to 85% of the cases [1,2].
Among the clinical manifestations of the disease, recurrent sinusitis, epistaxis, oral ulcers, otitis media, cough, hemoptysis, and dyspnea are reported, thus it must be differentiated from other pathological entities that could simulate this clinical spectrum; this causes difficulties in diagnosis, taking into account the wide possibility of etiologies. A mandatory differential diagnosis is Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis and one of the main causes of mortality in the world [3,4].
The association of pulmonary TB with GPA is, however, unusual. Establishing the definitive etiology when these two differential diagnoses are considered is difficult, considering that they share similar clinical, radiological, and histopathological features [5,6]. When considering this possible association, several scenarios arise:
• The coexistence of both diseases with a similar clinical spectrum
• The presence of ANCA vasculitis triggered by antigenic exposure to tuberculosis [7]
• The presence of ANCA antibodies in patients diagnosed with tuberculosis without pathogenic value [8]
• The high risk of tuberculosis infection in a patient who is receiving immunosuppression for the management of vasculitis [9]
Therefore, a case with the concurrence of both diseases is presented and discussed.
Case report
51 years-old, male, mestizo, resident in a city of northwest Colombia, mechanical technician.
Clinical findings
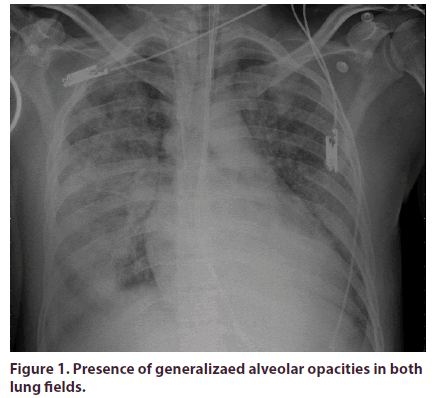
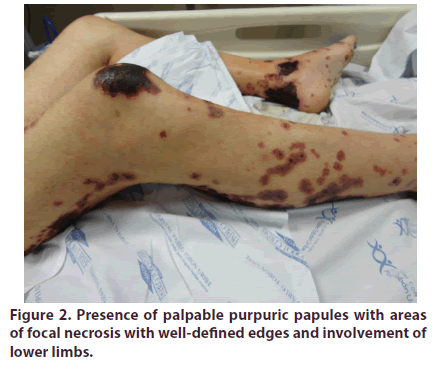
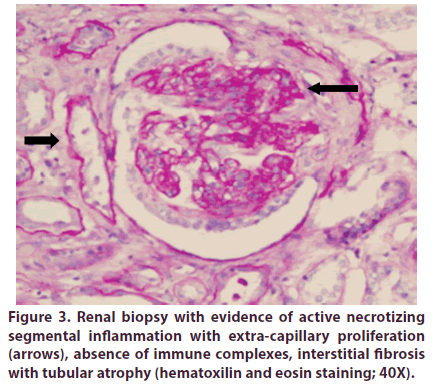
The patient didn´t have relevant family or psychosocial history. Healthy until August, 2012, when he consulted with a clinical picture of eight months of bilateral otalgia, initially diagnosed as acute otitis media, and treated with ventilation tubes without improvement. At two months, the patient presents symmetrical arthralgias in elbows, knees, and shoulders with inflammatory characteristics. One month later, he referred bilateral jaw and craniofacial pain with irradiation to the bitemporal region of severe intensity, tinnitus, and right hearing loss; a diagnosis of chronic mastoiditis was carried out, and simple mastoidectomy was performed. Thereafter, the patient presented persistent fever and headache. A diagnosis of meningeal tuberculosis was confirmed, initiating tetraconjugated treatment. Two months later, the patient presented a severe swallowing involvement that required gastrostomy, along with paralysis of vocal cords, severe neurological involvement with drowsiness, sensorineural hearing loss, and respiratory insufficiency, requiring transfer to the intensive care unit (Figure 1); during his stay, palpable purpuric papules that progressed to vesicles and ulcers with areas of focal necrosis appeared, with well-defined edges and involvement of lower and upper limbs, (Figure 2), as well rapidly-progressive glomerulonephritis and histological evidence of smallvessel vasculitis (Figure 3).
Figure 1: Presence of generalizaed alveolar opacities in both lung fields.
Figure 3: Renal biopsy with evidence of active necrotizing segmental inflammation with extra-capillary proliferation (arrows), absence of immune complexes, interstitial fibrosis with tubular atrophy (hematoxilin and eosin staining; 40X).
Diagnostic Approach
Diagnostic methods
They are highlighted in Table 1.
Table 1. Diagnostic methods.
| 2012 -2013 | 2019 | |
|---|---|---|
| Creatinine | 3.8 mg% (upper limit: 1mg%) | 1.32 mg% |
| Urinalysis | Hematuria, red blood cell casts; protein 150 mg | Normal |
| 24-hour proteinuria | 2.1 grams (upper limit: 150 mg) | 140 mg |
| Cerebrospinal fluid study | Proteins 81 mg%; glucose 59 mg%; 25 leukocytes with lymphocyte predominance | - |
| Autoimmunity | ||
| ANCA (ELISA) | PR3: 150 IU (0-20) | PR3: 0 IU |
| Infectious Diseases | ||
| Mycobacteria, fungi and aerobic bacteria culture in lung and meninges | Negative | |
| Tissue bacilloscopy (ear and mastoids) | Resistant acid-alcohol bacilli: Absent | |
| Mycobacterium Tuberculosis PCR (CSF) | Positive | |
| Pathology | ||
| Ear and mastoid biopsy | Chronic mastoiditis | |
| Lung biopsy | Presence of granulomas, Ziehl Neelsen staining: Positive | |
| Skin | Leukocytoclastic vasculitis | |
| Kidney | 16 glomeruli; 3 sclerosed Two with active necrotizing segmental inflammation; extra-capillary proliferation; absence of immune complexes; 30% interstitial fibrosis with tubular atrophy | |
| Imaging | ||
| Chest X-rays and tomography | Cavitated lesions in both lung fields | |
Diagnostic challenges
TB is a condition that can mimic many entities, specifically it represents a challenge to establish a definitive diagnosis when there are clinical data that could also suggest the coexistence or differential diagnosis of systemic vasculitis such as GPA, considering that they share clinical manifestations, including similar histopathological findings, such as evidence of granulomas. In turn, they are pathologies that may represent a vicious circle given by immunosuppression as a risk factor for presenting infection with Mycobacterium tuberculosis and the expression of molecules with antigenic potential in tuberculosis patients involved in the generation of autoantibodies.
Diagnostic reasoning
We faced a patient with a clinical picture of chronic course multisystem involvement: chronic otitis media, otomastoiditis, neurological, lung, and renal compromise, in whom infectious and autoimmune etiologies were considered within the diagnostic possibilities, However, when steroid removal was started after the diagnosis of meningeal TB, the patient presented deterioration in his clinical condition, with severe visceral organ involvement, which implied the use of immunosuppression (described below) and ruling out compromise due to systemic vasculitis. After an exhaustive follow-up and adequate response to immunosuppression, it was possible to conclude that this patient presented concomitantly both GPA and TB.
Therapeutic interventions, follow-up, and outcomes
The patient completed treatment with tetraconjugated antituberculosis treatment. Due to the described findings compatible with systemic vasculitis, treatment with pulses of methylprednisolone (500 mg IV qd for 3 days) and cyclophosphamide 300 mg IV as single dose were administered, without improvement in renal function. Seven days after, rituximab was started 1 gram IV fortnightly. At 1 month, the renal function improved (creatinine 3.8 mg% to 1.5 mg%), and rituximab was continued (500 mg IV every six months at a dose of 500 mg), until achieving complete remission in 2018. A relapse in February 2019 occurred, with fever and hemoptysis as cardinal symptoms (BVAS 13), so reinduction with rituximab was performed, with a posterior maintenance regimen of 500 mg IV every six months. In July 2019, rituximab was stopped due to Pneumocystis jirovecii pneumonia. Currently, the patient is on remission with methotrexate 10mg weekly and methylprednisone 8 mg PO qd.
Discussion
Granulomatous diseases are often difficult to diagnose because they can present in different forms, each with particular clinical and imaging features. Although the symptoms related to airway involvement may be the initial clinical manifestation of GPA, this compromise is, in most cases, indicative of a generalized disease [10], as in this case [11-13].
Several publications have suggested that numerous identifiable antibodies are found in this condition, and may be present in non-vasculitic diseases [14] and infections such as amebiasis, infective endocarditis, malaria, atypical pneumonia, mycosis, leptospirosis, poststreptococcal glomerulonephritis, onchocerciasis, chromomycosis, and blastomicosis, among others [15-18] Even some of these have been related with both tuberculous and non-tuberculous mycobacteria [17] , are known to induce the production of autoantibodies which are diagnostic markers of entities such as systemic vasculitis, playing a role that has not yet been elucidated in the pathogenesis of these diseases [5].
One of the main differential diagnoses to consider in this group of patients is TB, which can mimic many conditions, including GPA, and distinguishing between themh can be difficult, especially at the beginning of the disease [5]. The association of pulmonary TB with GPA is, however, unusual, although there are reports in the literature with the concomitant presentation of both [6,13].
As mentioned previously, the elevation of ANCA in serum has been described, secondary to infectious processes; in TB, this occurs [19]. The prevalence of ANCA in TB has been reported from 10-40%, the majority being p-ANCA [20]. A case-control survey conducted by Sherkat evaluated 32 patients with diagnosis of TB and 32 controls, finding P-ANCA positivity in 25% cases and 6.25% in controls, of which 75% were MPO-positive [21].
Different studies have been published which have attempted to analyze this relationship between ANCA and TB, finding a great diversity of associations. Sherkat et al. showed a relationship between anti-myeloperoxidase type with perinucleolar pattern (P-ANCA) with TB (p<0.01) [21]. Pradhan et al. reported the presence of ANCA in 30% of TB patients, in which there was also a correlation between P-ANCA (52.4%) and C-ANCA (38.1%) patients with TB, prevailing the anti-MPO pattern (47.6%) [5]. Texeira et al. also found similar data in their survey: up to 6% of TB patients had a P-ANCA pattern and 4% a C-ANCA pattern [18]. These discrepancies can be explained due to differences between demographic variables, autoimmune response, and antigenic exposure of each of the populations studied [18] along with previous exposure to different viruses, medications [22] and anti-tuberculosis drugs that can also alter the specificity of this test for the diagnosis of vasculitis [23].
On the other hand, the Bactericidal/Permeability Increasing Protein (BPI) that is closely related to Gram-negative infections, has also been described in mycobacteria by stimulation of TNFa. These infectious processes are involved in the development of BPIANCA, the latter activating neutrophils through the formation of immune complexes with BPI, which leads to the formation of NETs, which may play a role in the pathogenesis of vasculitis [7].
One of the keys to suspect a systemic vasculitis, in this case, was the absence of changes in the anti-PR3 titles after completing anti-tuberculosis treatment and the worsening of the clinical picture after withdrawal of glucocorticoids; in this situation, a suspicion of systemic vasculitis must be considered.
TB can produce secondary small-vessel vasculitis, with palpable purpura, erythema, urticaria, blisters, ulceration; it is caused by tissue damage secondary to deposition of immune complexes, endothelial invasión, the presence of self-reactive B and T lymphocytes, by direct injury to the vessel wall, along with hypersensitivity vasculitis [23]. The absence of positive cultures and the presence of kidney vasculitis make this differential diagnosis unlikely in this case.
Conclusions
Taking into account the inconclusive and highly heterogeneous data on the relationship between granulomatosis with polyangiitis and tuberculosis, some authors have made recommendations when faced with a scenario of diagnostic confusion:
• Always rule out tuberculosis in patients with positive ANCA
• The confirmatory diagnosis of vasculitis should not be based on the presence of positive ANCAs as the only finding; histopathological features should be taken into account and strictly correlated with the patient's clinic.
• Beyond the performance of ANCAs in the subgroup of patients with infectious diseases, we must take into account the presence of tuberculosis, given the interaction of TNF and the formation of BPIANCA with the consequent vasculitic activity
• Always wait for final reports on the culture tests since the histological findings of both (systemic vasculitis and tuberculosis) may include granulomatous reactions, vasculitis, and necrosis.
After doing a detailed review of the literature and exhaustive monitoring of the clinical course of this patient, and completing treatment for tuberculosis and receiving immunosuppressive management, we concluded that this subject presented concomitantly granulomatosis with polyangiitis and tuberculosis, understanding that patients with systemic vasculitis have predisposing risk factors mediated by immunosuppression to present opportunistic infections; in turn, some infectious processes such as tuberculosis can generate the expression of molecules, with the antigenic potential of generation of autoantibodies linked to systemic vasculitis.
References
- Roane DW, Griger DR. An Approach to Diagnosis and Initial Management of Systemic Vasculitis. Am. Fam. Physician. 60(5), 1421–1430 (1999).
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad. Med. J. 82(970), 483–488 (2006).
- Furin J, Cox H, Pai M. Tuberculosis. Lancet. 393(10181), 1642–1656 (2019).
- Guinn KM, Rubin EJ. Tuberculosis: Just the FAQs. mBio. 8(6), e01910–e01917 (2017).
- Pradhan VD, Badakere SS, Ghosh K et al. Spectrum of anti-neutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis overlaps with that of Wegener's granulomatosis. Indian. J. Med. Sci. 58(7), 283–288 (2004).
- Martínez-Martínez MU, Gómez-Gómez A, Oros-Ovalle C et al. A male patient with cavitary pulmonary disease. Reumatol. Clin. 9(1), 62–64 (2013).
- Takeda S, Watanabe-Kusunoki K, Nakazawa D et al. The Pathogenicity of BPI-ANCA in a Patient with Systemic Vasculitis. Front. Immunol. 10, 1334 (2019).
- Flórez-Suárez LF, Cabiedes J, Villa AR et al. Prevalence of antineutrophil cytoplasmic autoantibodies in patients with tuberculosis. Rheumatology (Oxford). 42(2), 223–229 (2003).
- Dorronsoro I, Torroba L. Microbiology of tuberculosis. An. Sist. Sanit. Navar. 30 (Suppl 2), 67–85 (2007).
- Santosham R, Deslauriers J. Tuberculosis and Other Granulomatous Diseases of the Airway. Thorac. Surg. Clin. 28, 155–161 (2018).
- Odman GC, Churg J. Wegener's granulomatosis. Arch. Pathol. 58, 533–553 (1954).
- Iijima Y, Kobayashi Y, Uchida Y et al. A case report of granulomatous polyangiitis complicated by tuberculous lymphadenitis. Medicine (Baltimore). 97(43), e12430 (2018).
- Molinari L, Melamud JI, Ferrari L et al. Granulomatosis of Wegener and tuberculosis a bad combination. Medicina (B Aires). 69(6), 640–642 (2009).
- Schmitt WH, van der Woude FJ. Clinical applications of antineutrophil cytoplasmic antibody testing. Curr. Opin. Rheumatol. 16, 9–17 (2004).
- Ardiles LG, Valderrama G, Moya P et al. Incidence and studies on antigenic specificities of antineutrophil cytoplasmic autoantibodies (ANCA) in poststreptococcal glomerulonephritis. Clin. Nephrol. 47, 1–5 (1997).
- Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Bull. Rheum. Dis. 47, 5–8 (1998).
- Medina F, Camargo A, Moreno J et al. Anti-neutrophil cytoplasmic autoantiboies in leprosy. Br. J. Rheumatol. 37, 270–273 (1998).
- Teixeira L, Mahr A, Jaureguy et al. Low seroprevalence and poor specificity of antineutrophil cytoplasmic antibodies in tuberculosis. Rheumatology. 44(2), 247–250 (2005).
- Esquivel-Valerio JA, Flores-Suárez LF, Rodríguez-Amado J et al. Antineutrophil cytoplasm autoantibodies in patients with tuberculosis are directed against bactericidal/permeability increasing protein and are detected after treatment initiation. Clin. Exp. Rheumatol. 28(Suppl 1), 35–39 (2010).
- Nava-Castaneda Á, Martín F, Voorduin S et al. Tuberculosis, granulomatosis with polyangiitis, ¿or both? Unusual clinical case. Arch. Soc. Esp. Oftalmol. 93(2), 101–104 (2018).
- Sherkat R, Mostafavizadeh K, Zeydabadi L et al. Antineutrophil cytoplasmic antibodies in patients with pulmonary tuberculosis. Iran. J. Immunol. 8, 52–55 (2011).
- Choi HK, Merkel PA, Walker AM et al. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis. Rheum. 43, 405–413 (2003).
- Carvalho M, Dominoni RL, Senchechen D et al. Cutaneous Leukocytoclastic Vasculitis Accompanied by Pulmonary Tuberculosis. J. Bras. Pneumol. 34(9), 745–748 (2008).