Case Report - Interventional Cardiology (2013) Volume 5, Issue 2
Treatment of inadvertent subintimal stenting during intervention of a coronary chronic total occlusion
- Corresponding Author:
- Emmanouil S Brilakis
Veterans Affairs, North Texas Healthcare System & University of Texas
Southwestern Medical Center, Dallas, TX, USA
Tel: +1 214 857 1547
Fax: +1 214 302 1341
E-mail: esbrilakis@yahoo.com
Abstract
Keywords
chronic total occlusion, complication, percutaneous coronary intervention
A 58-year-old man with hypertension and hyperlipidemia presented with a non-ST elevation myocardial infarction. He underwent coronary angiography, which revealed significant stenosis of the mid-left anterior descending artery and a chronic total occlusion (CTO) of the proximal right coronary artery (RCA) filling distally via left to right collaterals. He underwent successful intervention of the mid-left anterior descending artery; however, 1 month later, due to persistent angina refractory to medical therapy, he was referred for elective percutaneous coronary intervention (PCI) of his RCA CTO.
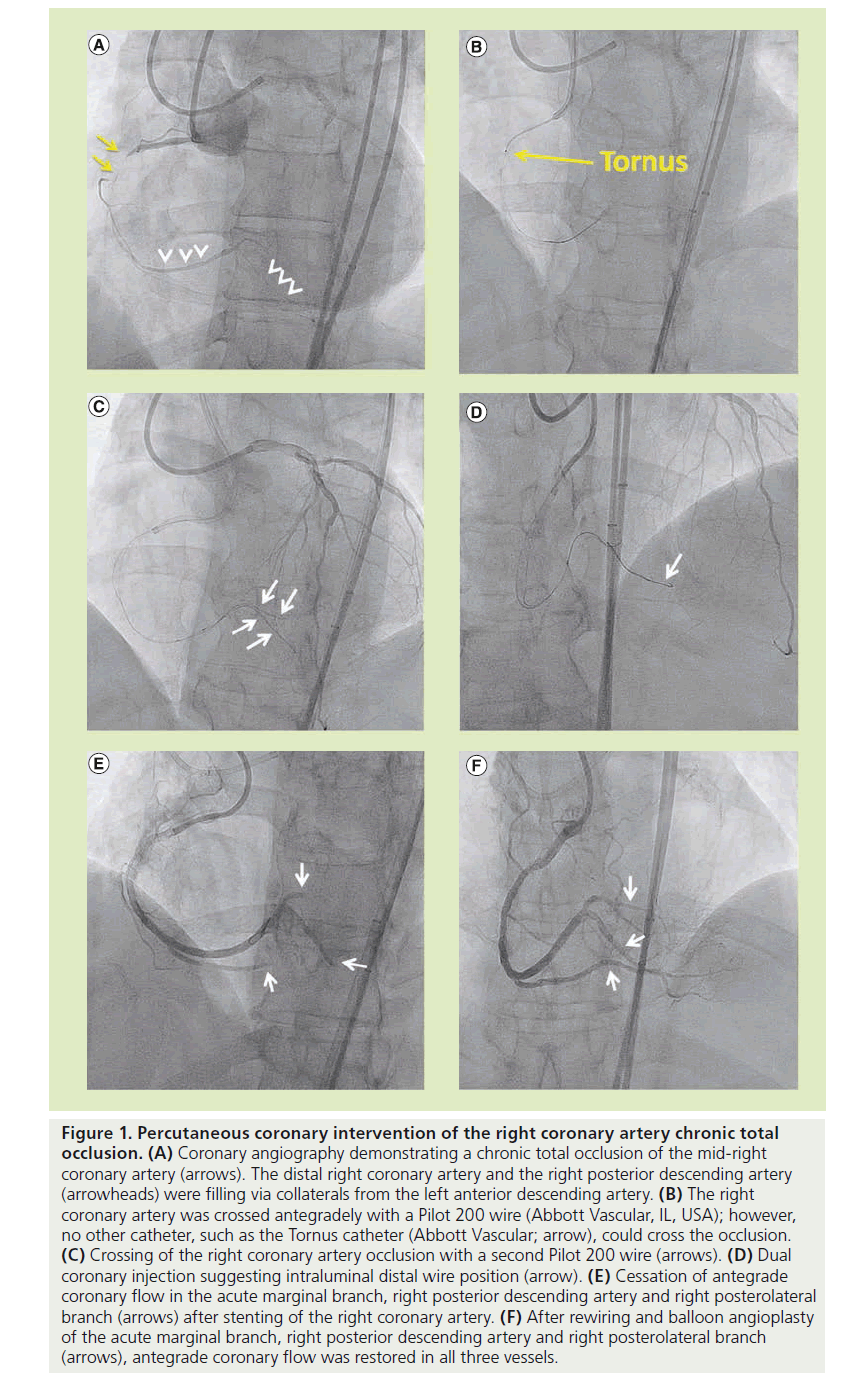
Bilateral femoral arterial access was obtained with 45-cm long sheaths. The left main and RCAs were engaged with a 7 French Extra Backup 3.5 (Cordis, NJ, USA) and a Judkins Right 4 (Cordis) guide catheter, respectively. Diagnostic angiography demonstrated a CTO of the proximal RCA (Figure 1A) with filling of the distal RCA and the right posterior descending artery (Figure 1A) via collaterals from the left anterior descending artery. Unfractionated heparin was administered for anticoagulation. The lesion was crossed in the antegrade direction with a Pilot 200 wire (Abbott Vascular, IL, USA); however, we were unable to advance any equipment through the proximal RCA, despite using multiple 1.5-mm balloons, a 2.1 and 2.6 French Tornus catheter (Abbott Vascular; Figure 1B), a CrossBoss catheter (Bridgepoint Medical, MN, USA), a FineCross™ catheter (Terumo Medical, NJ, USA) and 0.9-mm laser catheter (Spectranetics, CO, USA). The Judkins Right 4 guide was subsequently exchanged over the 0.014-inch Pilot 200 wire for an AL1 guide for additional support, but we were still unable to advance any equipment through the proximal RCA.
Figure 1: Percutaneous coronary intervention of the right coronary artery chronic total occlusion. (A) Coronary angiography demonstrating a chronic total occlusion of the mid-right coronary artery (arrows). The distal right coronary artery and the right posterior descending artery (arrowheads) were filling via collaterals from the left anterior descending artery. (B) The right coronary artery was crossed antegradely with a Pilot 200 wire (Abbott Vascular, IL, USA); however, no other catheter, such as the Tornus catheter (Abbott Vascular; arrow), could cross the occlusion. (C) Crossing of the right coronary artery occlusion with a second Pilot 200 wire (arrows). (D) Dual coronary injection suggesting intraluminal distal wire position (arrow). (E) Cessation of antegrade coronary flow in the acute marginal branch, right posterior descending artery and right posterolateral branch (arrows) after stenting of the right coronary artery. (F) After rewiring and balloon angioplasty of the acute marginal branch, right posterior descending artery and right posterolateral branch (arrows), antegrade coronary flow was restored in all three vessels.
We subsequently advanced a second Pilot 200 wire in a ‘parallel wire’ fashion antegradely through the CTO into the distal RCA (Figure 1C). With increased backup support from the AL1 guide and a second parallel wire, an intravascular ultrasonography catheter was able to be advanced further into the RCA. A contralateral injection (Figure 1D) suggested an intraluminal distal wire position. After predilation, four overlapping XIENCE V® stents (Abbott Vascular; 2.5 × 28, 2.5 × 28, 2.75 × 28 and 3 × 28 mm) were deployed from the distal to proximal RCA; however, post-stenting angiography revealed occlusion of the acute marginal branch, the posterior descending artery and the right posterolateral branch (Figure 1E), suggesting subintimal stent deployment. The patient developed chest pain and inferior ST-segment elevation.
The distal tip of a Pilot 200 guidewire was shaped into a 90° bend and the wire was aggressively advanced through each side branch, using a penetration technique, successfully entering the distal true lumen of all three vessels. After multiple sequential balloon dilations with 1.5- and 2.0-mm balloons, antegrade thrombolysis in myocardial infarction 3 flow was restored in all three vessels with resolution of the chest pain and electrocardiographic changes (Figure 1F). The patient had a periprocedural myocardial infarction with a peak creatine kinase-MB level of 41 ng/ml, but otherwise the patient had an uncomplicated recovery with resolution of his angina.
Discussion
■Crossing of the balloon-uncrossable CTO
The most common reason for CTO PCI failure is failure to cross the lesion with a guidewire [1]. The second most common reason is the inability to cross the lesion with a balloon after successful guidewire crossing (balloon uncrossable CTOs) [1,2]. Crossing of the balloon uncrossable CTO can be achieved using several techniques [2]. First, a small (1.25 or 1.5 mm) balloon is inserted as deep as possible into the CTO and inflated at high pressure. Long balloons are preferred as the highest profile segment of the balloon is the mid-shaft marker. If this fails, the balloon can be inflated again until it ruptures, which can modify the plaque and allow lesion crossing. If this fails, the Tornus catheter (Asahi Intecc, Japan) can be utilized. The Asahi Tornus device is a wire-braided microcatheter designed to penetrate otherwise difficult to cross CTO lesions by advancing using counterclockwise rotation. Second-line techniques include the use of strategies that increase guide catheter support, such as using a larger and more supportive shape guide catheter, using the Guideliner catheter (Vascular Solutions, MN, USA) [3] or various anchor balloon techniques [4]. Occasionally, a combination of techniques, such as Anchor-Tornus [5] or Guideliner-Tornus may be required. Third-line strategies include the use of rotational atherectomy or laser, or recrossing the lesion through a different path, as was ultimately done in our case.
■Confirming intraluminal wire position
After antegrade CTO crossing, it is of paramount importance to confirm that the wire has entered the distal true lumen before proceeding with balloon dilation and stenting. This can be accomplished by dual injection, contrast injection through a microcatheter, using intravascular imaging and by observing the wire movement into distal branches. Dual injection is most commonly used and is crucial for nearly all CTO procedures, even when most collaterals are ipsilateral, as ipsilateral collaterals may become occluded during crossing attempts [6,7]. We recommend against using antegrade contrast injection through a microcatheter, because it carries the risk of subintimal space ‘staining’ and dissection propagation if the wire is not in the distal true lumen, which can then hinder subsequent true lumen re-entry attempts. Intravascular imaging is another option if the imaging catheter can cross the lesion. Intravascular ultrasonography is usually used [8], although optical coherence tomography has also been reported [9]. However, advancing the imaging catheter distally may be challenging. Moreover, the forceful contrast injection required for optical coherence tomography may propagate a subintimal dissection. Although distal side branch entry of the wire is suggestive of true lumen position [10], it may also be misleading as the wire can advance subintimally into side branches.
If the subintimal guidewire position is confirmed, entering the distal true lumen can be achieved using several strategies [11]: in the original subintimal tracking and re-entry (STAR) technique, the knuckled guidewire was advanced distally until it spontaneously entered into the distal true lumen [12]; in the more contemporary limited antegrade subintimal tracking (LAST) or mini-STAR [13] technique, the area of subintimal dissection is limited by re-entering the true lumen as close as possible to the distal cap without propagating the dissection into the distal part of the vessel; finally, the Stingray system (Bridgepoint Medical) has been specifically designed to facilitate distal true lumen crossing. The Stingray balloon is 2.5 mm in diameter and 10 mm in length and has a flat shape with two side exit ports: upon low-pressure inflation (2–4 atm) it orients one exit port automatically towards the true lumen. The Stingray wire is a stiff guidewire that has a 20-cm distal radiopaque segment and a 0.009-inch tapered tip with a 0.0035-inch distal taper. The Stingray guidewire is directed towards one of the two side ports of the Stingray balloon under fluoroscopic guidance to re-enter the distal true lumen [14,15].
■Distal vessel rescue after subintimal stenting
Once stents have been inadvertedly deployed in the subintimal space without connection to the distal true lumen, the patient may remain asymptomatic [16,17] or may develop ST-segment elevation due to side branch loss, as in our case. Various techniques can be used to regain access into the distal true lumen. First, antegrade crossing can be attempted: in our case we were able to advance a stiff, polymer-jacketed guidewire (Pilot 200) into each of the occluded branches (acute marginal branch, posterior descending artery and right posterolateral branch). Balloon angioplasty allowed the restoration of antegrade flow in each of these branches. An alternative option is to use a dedicated re-entry system, such as the Stingray wire and balloon (Bridgepoint Medical) [14,15] or the Venture catheter (St Jude Medical, MN, USA) [18,19]; however, difficulty may be encountered in advancing the catheter past the stented segment. Third, retrograde crossing into the true lumen can be attempted, especially in the presence of large and nontortuous collateral vessels [7]. These same techniques can also be employed to re-enter the true lumen in cases of inadvertent diagnostic or guide catheter dissection during vessel engagement [20].
Apart from subintimal stenting, antegrade flow can be slow after CTO PCI because of distal edge dissection, distal embolization, vasoconstriction and competitive flow from collaterals. Edge dissections are detected in 1.7% of cases by angiography versus 9.2% of cases by intravascular ultrasonography [21]. Approximately half of the dissections occur in the proximal and the distal edge [22]. CTO lesions can be complex and calcified, increasing the risk for edge dissection [21], and this possibility must be considered if thrombolysis in myocardial infarction flow is reduced after stenting. No reflow and distal embolization usually occurs in patients with acute coronary syndromes or saphenous vein graft stenting, but can at times occur after CTO PCI due to embolization of atheromas, platelet– fibrin complexes, cholesterol crystals and macrophages [23,24]. Vasospasm is always a possibility after CTO PCI, especially when the distal vessel is of small caliber and diffusely diseased, but usually resolves after nitroglycerin administration. Finally, in some cases with well-developed collateral circulation, contralateral blood flow remains brisk after stenting, competing with antegrade flow, but this can be elucidated with more forceful antegrade injections.
Conclusion
In summary, inadvertent subintimal space stenting during CTO PCI can be salvaged using a stiff, polymer-jacketed guidewire to gain entry into the distal true lumen. Routine application of preventive strategies and awareness of potential treatment options can minimize the occurrence and the consequences of this CTO PCI complication.
Future perspective
Failure to cross is the most common cause of CTO PCI failure. Ensuring that the coronary guidewire enters into the distal true lumen is of paramount importance to prevent inadvertent subintimal stenting. Careful review of coronary angiography using dual injection is currently the main method of ensuring distal true lumen guidewire location, but novel equipment and techniques are constantly being developed to facilitate this task and make CTO PCI safer and more effective.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient for the inclusion of their medical and treatment history within this case report.
Financial & competing interests disclosure
S Banerjee has received research grants from Gilead and Boston Scientific; has received consultant/speaker honoraria from Covidien and Medtronic; and has ownership in MDCARE Global and intellectual property in HygeiaTel. E Brialkis has received speaker honoraria from St Jude Medical, Terumo and Bridgepoint Medical and a research grant from Guerbet; his spouse in an employee of Medtronic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Chronic total occlusion guidewire crossing
▪ Successful chronic total occlusion guidewire crossing to the distal lumen remains the most common reason for chronic total occlusion percutaneous coronary intervention failure.
▪ Failure to dilate the lesion after successful guidewire crossing is the second most common reason for chronic total occlusion percutaneous coronary intervention failure.
Confirming intraluminal wire position
▪ Intraluminal wire position can be confirmed using contralateral injection, microcatheter contrast injection, intravascular ultrasonography or observation of wire movement into distal branches.
Distal vessel rescue
▪ Distal vessel jailing after inadvertent subintimal stenting or dissection can be treated using stiff polymer-jacketed wiring of the distal true lumen, dedicated re-entry devices or retrograde crossing into the true lumen.
References
- Stone GW, Reifart NJ, Moussa I et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation 112(16), 2530–2537 (2005).
- Brilakis ES, Banerjee S. Crossing the ‘balloon uncrossable’ chronic total occlusion: Tornus to the rescue. Cathet. Cardiovasc. Interv. 78(3), 363–365 (2011).
- Luna M, Papayannis A, Holper EM, Banerjee S, Brilakis ES. Transfemoral use of the GuideLiner catheter in complex coronary and bypass graft interventions. Catheter Cardiovasc. Interv. 80(3), 437–446 (2012).
- Di Mario C, Ramasami N. Techniques to enhance guide catheter support. Catheter Cardiovasc Interv 72(4), 505–512 (2008).
- Kirtane AJ, Stone GW. The Anchor-Tornus technique: a novel approach to ‘uncrossable’ chronic total occlusions. Catheter Cardiovasc. Interv. 70(4), 554–557 (2007).
- Singh M, Bell MR, Berger PB, Holmes DR Jr. Utility of bilateral coronary injections during complex coronary angioplasty. J. Invasive Cardiol. 11(2), 70–74 (1999).
- Brilakis ES, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 5(4), 367–379 (2012).
- Banerjee S, Master R, Brilakis ES. Intravascular ultrasound-guided true lumen re-entry for successful recanalization of chronic total occlusions. J. Invasive Cardiol. 22(12), 608–610 (2010).
- Schultz C, van der Ent M, Serruys PW, Regar E. Optical coherence tomography to guide treatment of chronic occlusions? J. Am. Coll. Cardiol. Interv. 2(4), 366–367 (2009).
- Hussain F. Distal side branch entry technique to accomplish recanalization of a complex and heavily calcified chronic total occlusion. J. Invasive Cardiol. 19(11), E340–E342 (2007).
- Michael TT, Papayannis AC, Banerjee S, Brilakis ES. Subintimal dissection/reentry strategies in coronary chronic total occlusion interventions. Circ. Cardiovasc. Interv. 5(5), 729–738 (2012).
- Colombo A, Mikhail GW, Michev I et al. Treating chronic total occlusions using subintimal tracking and reentry: the STAR technique. Catheter Cardiovasc. Interv. 64(4), 407–411; discussion 412 (2005).
- Galassi AR, Tomasello SD, Costanzo L et al. Mini-STAR as bail-out strategy for percutaneous coronary intervention of chronic total occlusion. Catheter Cardiovasc. Interv. 79(1), 30–40 (2012).
- Werner GS. The BridgePoint devices to facilitate recanalization of chronic total coronary occlusions through controlled subintimal reentry. Expert Rev. Med. Devices 8(1), 23–29 (2011).
- Brilakis ES, Badhey N, Banerjee S. ‘Bilateral knuckle’ technique and Stingray re-entry system for retrograde chronic total occlusion intervention. J. Invasive Cardiol. 23(3), E37–E39 (2011).
- Omurlu K, Ozeke O. Side-by-side false and true lumen stenting for recanalization of the chronically occluded right coronary artery. Heart Vessels 23(4), 282–285 (2008).
- Krivonyak GS, Warren SG. Compression of a subintimal or false lumen stent by stenting in the true lumen. J. Invasive Cardiol. 13(10), 698–701 (2001).
- Badhey N, Lombardi WL, Thompson CA, Brilakis ES, Banerjee S. Use of the venture wire control catheter for subintimal coronary dissection and reentry in chronic total occlusions. J. Invasive Cardiol. 22(9), 445–448 (2010).
- Iturbe JM, Abdel-Karim AR, Raja VN, Rangan BV, Banerjee S, Brilakis ES. Use of the venture wire control catheter for the treatment of coronary artery chronic total occlusions. Catheter Cardiovasc. Interv. 76(7), 936–941 (2010).
- Martinez-Rumayor AA, Banerjee S, Brilakis ES. Knuckle wire and stingray balloon for recrossing a coronary dissection after loss of guidewire position. JACC Cardiovasc. Interv. 5(10), e31–e32 (2012).
- Biondi-Zoccai GG, Agostoni P, Sangiorgi GM et al. Incidence, predictors, and outcomes of coronary dissections left untreated after drugeluting stent implantation. Eur. Heart J. 27(5), 540–546 (2006).
- Liu X, Tsujita K, Maehara A et al. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc. Interv. 2(10), 997–1004 (2009).
- Kotani J, Nanto S, Mintz GS et al. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation 106(13), 1672–1677 (2002).
- Katsuragawa M, Fujiwara H, Miyamae M, Sasayama S. Histologic studies in percutaneous transluminal coronary angioplasty for chronic total occlusion: comparison of tapering and abrupt types of occlusion and short and long occluded segments. J. Am. Coll. Cardiol. 21(3), 604–611 (1993).