Review Article - Interventional Cardiology (2015) Volume 7, Issue 2
Transcatheter therapies for arrhythmias in patients with complex congenital heart disease
- Corresponding Author:
- Charles I Berul
Division of Cardiology, Children’s National Health System 111 Michigan Avenue
NW, Washington, DC 20010 USA
Tel: +1 202 476 2020
Fax: +1 202 476 5700
E-mail: cberul@childrensnational.org
Abstract
Arrhythmias are responsible for significant morbidity and mortality in patients with complex congenital heart disease (CHD). Pharmacologic antiarrhythmic therapy often proves ineffective or intolerable due to adverse side effect profile in a patient group consigned to lifelong treatment. Interventional electrophysiology procedures have revolutionized arrhythmia management in the CHD population since the introduction of transcatheter ablation in the late 1980s. Tremendous advances in electroanatomic mapping and ablation technology have led to increased procedural success in conjunction with a dramatically reduced risk profile to overcome the challenges of patient size, abnormalities of the intrinsic conduction system, mapping of arrhythmia in complex congenital cardiac malformations and limited access to surgically isolated cardiac chambers. Future interventional electrophysiology innovations are geared to provide further reduction in radiation exposure for the CHD population.
Keywords
Ablation, arrhythmia, congenital heart disease, electrophysiology
Congenital heart disease (CHD) affects approximately 1% of live births worldwide. Complex lesions include tetralogy of Fallot (TOF), transposition of the great arteries, congenitally corrected transposition of the great arteries, Ebstein’s anomaly and single ventricle defects such as hypoplastic left heart syndrome or tricuspid atresia. These complex defects occur much less frequently than simple shunt or obstructive lesions although they are associated with significant morbidity and require lifelong specialist cardiac care [1]. Advances in cardiac imaging, transcatheter interventions and cardiac surgery in the past 50 years have led to a dramatic increase in survival rates for those affected by complex CHD shifting the mortality burden from infancy to adulthood [2]. The incidence of arrhythmia in those with CHD is considerably higher than that of the general population, particularly in adults with CHD, and accounts for a significant proportion of the overall morbidity and mortality [3]. Even in simple, isolated congenital defects such as a moderate secundum atrial septal defect (ASD), there is significant risk of arrhythmia development by the third decade of life and the risk continues for many decades following ASD repair [4]. It is difficult to estimate the exact prevalence of arrhythmias in the CHD population due to heterogeneity of the CHD subtypes, variability in reporting and inconsistent availability of specialized congenital follow-up care. Adults who have undergone TOF repair during childhood comprise a significant proportion of the complex adult CHD population. Of these adults with repaired TOF, 43% had a sustained arrhythmia or had undergone arrhythmia intervention at a median age of 37 years [5]. Pharmacologic therapy has frequent side effects and limited efficacy in controlling arrhythmias associated with structural heart disease. Interventional procedures are therefore becoming standard, in some cases first line, therapy for potentially definitive management of rhythm disturbances. This article will review the burden of arrhythmias in complex CHD and techniques to overcome the challenges created by altered congenital and post-surgical cardiac anatomy in the mapping and targeting of arrhythmogenic tissue in patients with CHD. Though they have had a remarkable positive impact on management of rhythm disturbances in the complex CHD population, this review will not cover implantable cardioverter defibrillators or pacemaker technologies.
Arrhythmias in complex CHD
Congenital anomalies of the conduction system Multiple mechanisms contribute to the development of arrhythmias in CHD (Table 1). Abnormal cardiac embryology responsible for structural cardiac malformations also alters development of the conduction system [6]. Nowhere is this more apparent than in heterotaxy syndrome where abnormalities of visceroatrial situs are associated with extremely complex cardiac defects. Isomerism of the right atrium may result in residual twin atrioventricular (AV) nodes whereas isomerism of the left atrium may result in hypoplasia or absence of the AV node [7]. Residual twin AV nodes may be the substrate for development of complex AV nodal reentry tachycardia or an AV reciprocating tachycardia between the two nodes, or sometimes both mechanisms. Ablation of one of the AV nodes may be necessary to treat medically refractory supraventricular arrhythmia in this instance [8,9]. It is critical for the congenital electrophysiologist to define the functionality of the dominant AV node to ensure sufficient conduction following ablation. Abnormal ventricular looping seen in L-transposition of the great arteries may also result in congenital or postnatal AV block, accessory AV node tissue and abnormal course of the bundle branches [10]. The location of the AV node and bundle of His are determined by the position of the right and left ventricles and associated AV ring. In L-transposition of the great arteries, the AV node is anteriorly displaced and the bundle of His passes anteriorly to the ventricular septal defect [11]. In contrast, the AV node in tricuspid atresia is located in the inferior right atrium and the AV conduction system courses inferiorly and posteriorly to the ventricular septal defect. Electrophysiology studies in patients with Ebstein’s malformation also require careful delineation of the AV groove to identify the AV node and bundle of His since the anatomical positions, altered by inferior displacement of the tricuspid valve and atrialization of the right ventricle, are often difficult to define. In addition Ebstein’s malformation with Wolff–Parkinson–White syndrome is frequently associated with multiple accessory pathways that may have antegrade, retrograde or bidirectional conduction [12]. These are often challenging for catheter ablation therapy due to the anatomic distortion of the tricuspid annulus and the variability of accessory pathway anatomy and physiology.
Table 1. Arrhythmia type and mechanisms associated with unrepaired and repaired CHD
Physiologic & post-surgical consequences of CHD
The effects of chronic pressure and volume loads either from congenital anomalies or secondary to post-surgical anatomy creates physiologic changes in myocardial architecture which, combined with the anatomic scars and functional barriers, increase the risk of arrhythmia development. For example, adults with TOF likely underwent surgical repair in the era of transannular patch repair of the right ventricular outflow tract thus creating free pulmonary regurgitation, chronic right ventricular volume overload, progressive dilation and dysfunction and ultimately potentially fatal ventricular arrhythmias [3]. Localized myocardial ischemia secondary to coronary anomalies or fibrosis at surgical scar sites also contribute to the combined increased risk of rhythm disturbances, particularly ventricular arrhythmias, in those with CHD. Surgical repair of CHD risks damage to the intrinsic components of the conduction system increasing susceptibility to atrial and/or ventricular arrhythmias. All of these mechanisms contribute to the risk of ventricular tachyarrhythmias in TOF. The sinoatrial node and associated arterial supply is particularly vulnerable to damage or obliteration in congenital heart surgeries involving the superior vena cava/right atrial junction, such as the modified bidirectional Glenn surgery for single ventricle palliation or repair of anomalous pulmonary venous drainage to the superior vena cava which both involves division of the cavoatrial junction. Suture lines, for example, from a Mustard or Senning type atrial baffle for D-transposition of the great arteries, also disrupt the conduction system. Even cannulation for cardiac bypass can impair sinus node function.
Clinical impact of arrhythmias associated with CHD
Paroxysmal supraventricular tachycardia (SVT) is the most commonly seen arrhythmia in the pediatric population with structurally normal hearts. SVT is also relatively common in the CHD population but may be less well tolerated than in those with structurally normal hearts, resulting in syncope or hemodynamic instability. Patients with CHD are also more likely to develop a wide variety of arrhythmia type much earlier than the more typical age range of presentation, such as atrial fibrillation in patients with premature atrial dilation secondary to post-surgical anatomy and physiology. Most CHD patients have multifactorial etiology of their rhythm disturbances and can develop different arrhythmia types during their lifetime. A patient with hypoplastic left heart syndrome may develop an automatic tachycardia such as ectopic atrial tachycardia in the neonatal period, junctional ectopic tachycardia in the postoperative setting, sinus node dysfunction in the teenage years and late onset of reentrant atrial tachycardia such as intra-atrial reentrant tachycardia from atrial dilation or ventricular arrhythmias in adulthood. The burden of arrhythmia in those with CHD is associated with debilitating symptoms, greatly increases the risk of sudden death and is estimated to account for approximately 20% of the total mortality in patients with complex congenital cardiac lesions [3]. Effective therapeutic options for arrhythmia management are therefore essential to improving the quality of life and long-term survival of patients with CHD.
Indications for arrhythmia ablation
The indications for catheter ablation of arrhythmias in the CHD population are similar to the non-CHD population including significant symptoms, syncope or hemodynamic instability particularly when there has been failure or intolerance of medical therapy. Antiarrhythmic medications, such as beta blockers, are frequently contra-indicated in the complex CHD population due to their negative inotropic effects, particularly in the post cardiac surgery setting. All antiarrhythmic medications can have pro-arrhythmic side effects, particularly in patients with ischemic heart conditions, myocardial scars and/or diminished ventricular function – a combination that may be seen in repaired CHD. These medications are often poorly tolerated in teenage patients in whom the challenges of compliance are heightened. We have had multiple patients re-present with recurrence of life-threatening arrhythmias secondary to medication noncompliance in adolescence. Previously, the consensus recommendations were to try medications as first-line and often second- and third-line approach despite poor efficacy of chronic antiarrhythmic pharmacologic therapy prior to interventional procedures. As success rates and risk reduction have improved with advances in technology, the threshold to perform an ablation has become lower. The risk-to-benefit equation of ablation versus medical therapy is carefully weighed in the pediatric and CHD populations, and has resulted in arrhythmia ablation becoming an accepted first-line therapy in many circumstances such as IART following Fontan palliation in adults without significant comorbidities [13–16]. Arrhythmia ablation should also be considered in preoperative planning prior to cardiac surgery to reduce postoperative risk and to perform the procedure prior to surgical isolation of cardiac chambers or anatomic locations.
Challenges of intervention in the complex CHD population
Prediction of arrhythmia substrate location in CHD
The aforementioned intrinsic variants in the anatomy of the conduction system in complex CHD are associated with abnormalities in baseline electrocardiogram findings. These pose additional challenges in deciphering the location of suspected accessory pathways and arrhythmia substrates. The CHD population are known to have a high rate of both true pre-excitation (due to accessory pathways) and pseudo-pre-excitation (the appearance of a delta wave on electrocardiogram that may be secondary to abnormal cardiac structure without an accessory pathway) [17]. Different algorithms are often required to predict the location of arrhythmia circuits and accessory pathways in those with complex CHD anatomy, compared with their utility in ‘standard’ adults with structurally normal hearts [18]. Great strides in 3D mapping system development (as illustrated in Figure 1) and advanced catheter technologies over the last few years have been particularly useful for the complex CHD and post-cardiac surgery population in whom the location of arrhythmia substrate and its proximity to important structures differs from those with structurally normal hearts. The risk of disruption of normal conduction can be mitigated by knowledge of the intrinsic anatomy and delineation of the arrhythmia substrate in relation to the bundle of His and AV node.
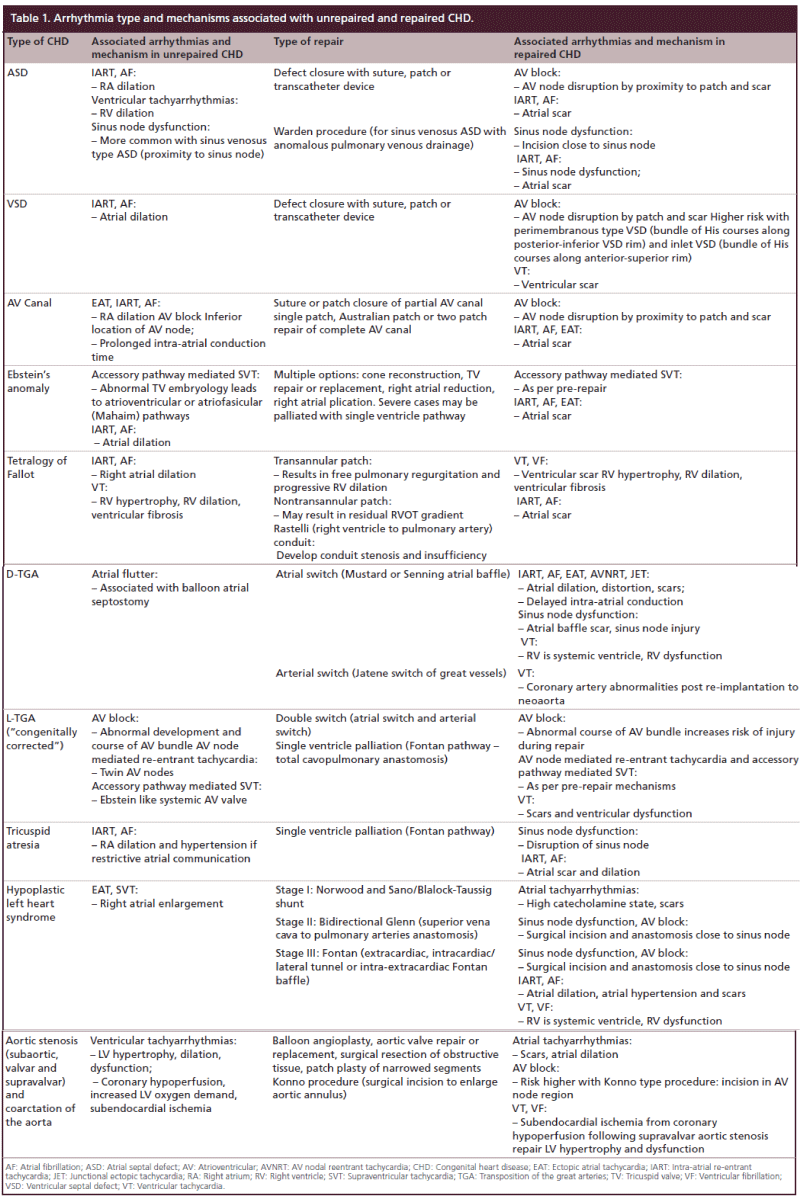
Figure 1: Successful radiofrequency ablation of recurrent ectopic atrial tachycardia, refractory to pharmacologic therapy, in a 15-month old, 6 kg, child status post-complete atrioventricular canal and mitral valve repair (for parts [A & B], see above; for parts [C & D], see facing page). (A) Baseline 15-lead electrocardiogram with atrial and ventricular rate of 200 beats per minute, ectopic atrial tachycardia with left axis deviation and right bundle branch block. The electrophysiology study and ablation procedure was performed with a 7-Fr 4-mm tipped EZ steer NAV D-F mapping and ablation catheter (Biosense Webster Inc, Diamond Bar, CA, USA) and a 5-Fr esophageal bipolar pacing and recording catheter (Cardio Command, Inc, Tampa, FL, USA). (B) Surface and intracardiac electrograms with the premature atrial contraction (PAC) highlighted by the arrow. There is early activation on the ablation catheter relative to the PAC onset. (C) Electroanatomic propagation map of premature atrial contractions in the right lateral view with successful ectopic atrial tachycardia radiofrequency ablation performed in the posterior high right atrium at the azygous vein orifice. (Red dots: radiofrequency ablation sites; gray dots: sites of no electrical signal in the systemic veins). Total fluoroscopy time was 3 min and area dose product was 48.7 uGym2. (D) Postablation procedure 15-lead electrocardiogram with atrial and ventricular rate of 96 beats per minute, normal sinus rhythm, left axis deviation and right bundle branch block. IVC: Inferior vena cava; RA: Right atrium; SVC: Superior vena cava; TV: Tricuspid valve.
Intra-atrial reentrant tachycardia (IART) encompasses the atrial macroreentrant circuits frequently seen in the adult CHD population. IART is a stereotypical arrhythmia mechanism that includes classic cavotricuspid isthmus related atrial flutter and atypical circuits associated with post-surgical scar tissue and islands of heterogeneous conduction properties [19]. Patients who have undergone Mustard or Senning type atrial switch procedures for d-transposition of the great arteries, Fontan procedure (Figure 2) for single ventricle palliation or repair of TOF are most at risk of IART development secondary to progressive atrial dilation and wall thickening. The IART circuit may loop around in the atria secondary to scar tissue requiring multiple ablation points to create an ablation line that interrupts the circuit to successfully terminate the reentrant arrhythmia [20]. In a single center study of 95 patients with IART and TOF or similar anatomic diagnoses such as double outlet right ventricle, IART circuits had critical areas for ablation at the cavotricuspid isthmus in 53%, the lateral right atrial wall in 32%, the remainder of the right atrium in 12% and the left atrium in 3% [21]. Accessibility to the tricuspid isthmus location should be considered prior to the electrophysiology procedure in order to plan transbaffle puncture if necessary for patients who have undergone Mustard, Senning or Fontan procedures [20]. A similar strategy of mapping scar tissue is employed in the ventricle to determine ventricular tachycardia foci and appropriate ablation sites for example in patients who have undergone transannular patch repair of TOF [22]. When clinical ventricular arrhythmias cannot be reproduced during electrophysiology studies, substrate mapping allows for planning anatomic targets for ablation in CHD patients. Understanding of both the congenital anatomy and the surgical procedures is vital to safely and effectively performing substrate- based catheter ablation, while avoiding injury to normal conduction tissue.
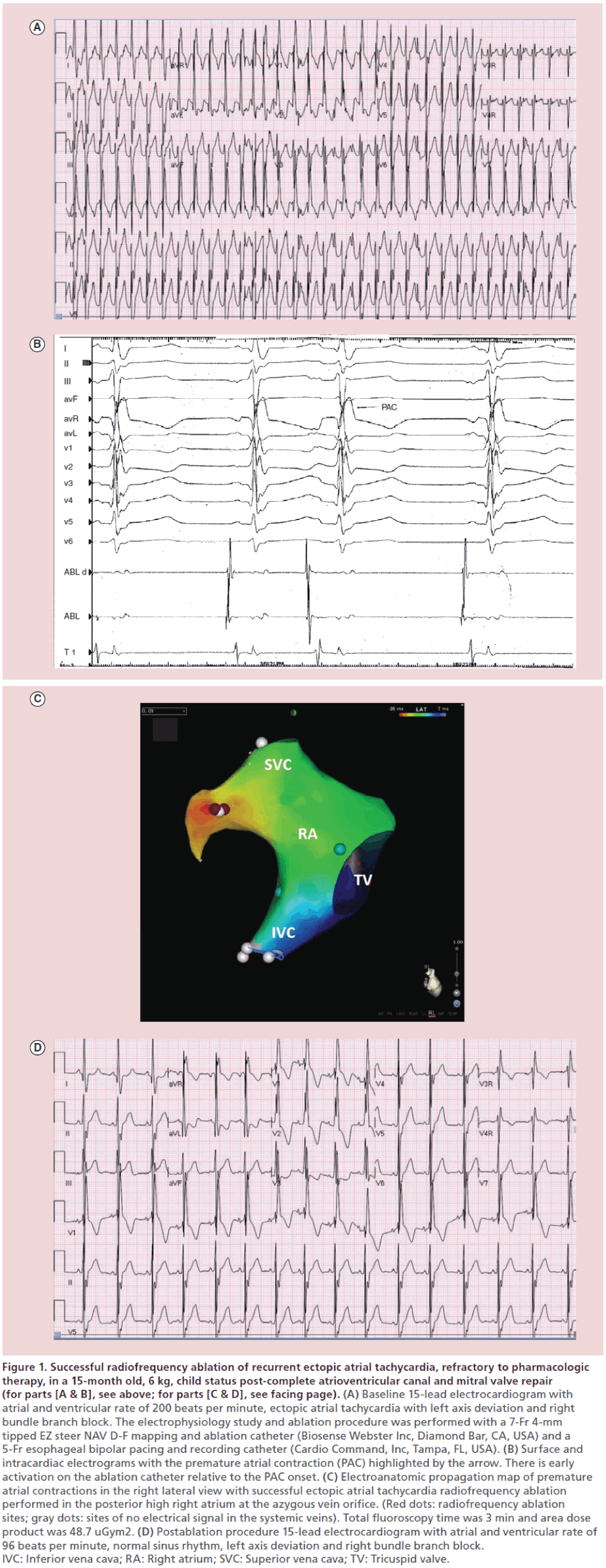
Figure 2: Cardiac catheterization and electrophysiology study in an 18-year-old male patient with recurrent intraatrial reentry tachycardia status post a nonfenestrated lateral tunnel Fontan for single ventricle palliation of tricuspid atresia. (A) Lateral tunnel Fontan baffle angiogram outlined in light green in the straight AP projection (*: transbaffle puncture site). (B) CARTO® electroanatomic map in sinus rhythm of the lateral tunnel Fontan baffle and the atria (outlined in white) in the AP projection. To facilitate electrophysiology study and mapping of the atria a transbaffle puncture was performed with an 8-Fr Mullins sheath under fluoroscopic and echocardiographic guidance. Nonsustained IART was induced during the case although terminated prior to completion of mapping. Scar substrate mapping revealed a large scar in the lateral tunnel portion of the right atrium and a radiofrequency ablation line of block from the scar in the anterior–superior to the IVC was performed (*: transbaffle puncture site; red dots: radiofrequency ablation points; yellow dots: His markers). The patient had no further IART at last follow-up. IVC: Inferior vena cava; LPA: Left pulmonary artery; RPA: Right pulmonary artery; SVC: Superior vena cava.
Access to chambers for mapping & ablation of arrhythmia
Congenital cardiac and post-surgical anatomy poses challenges in accessing cardiac chambers to map and ablate arrhythmia substrates. In those with structurally normal hearts, the technique of transseptal puncture with a Brockenbrough needle to access the left atrium and pulmonary veins from the systemic venous approach transseptal has been augmented by improvements in image guidance. The option of radiofrequency perforation has been reported to be successful in cases when traditional needle puncture has failed [23]. These techniques are also useful in the complex CHD population although the procedure remains immensely more challenging due to abnormal deviation of the atrial septum and post-surgical atrial anatomy. While transesophageal, transthoracic or intracardiac echocardiography may provide adequate guidance for transseptal perforation in the complex CHD population, additional imaging may assist in safer transseptal passage. The risk profile and length of the procedure may be ameliorated by cardiac anatomy image fusion from echocardiography, MRI, CT or fluoroscopy with the 3D electroanatomic mapping system [24].
Challenges remain in accessing cardiac chambers for patients who have undergone Fontan palliation, a group who are at particularly high risk of developing refractory atrial tachycardias. The Fontan procedure is usually preceded by a Hemi-Fontan or bidirectional Glenn surgery performed in the first year of life, involving anastomosis of the superior cava directly to the pulmonary arteries and oversewing of the pulmonary valve. Completion of the Fontan procedure, usually by 2–3 years of life, involves baffling of the inferior vena cava to the pulmonary arteries thus creating a circuit of passive systemic venous blood flow directly to lungs (Figure 2). If a Fontan fenestration has been created between the baffle and atrial chambers at the time of surgery and is still patent, or if there is a baffle leak, mapping and ablation catheters can be advanced from the systemic veins through the fenestration or baffle leak to the atria. However, in many cases a fenestration has not been created, has closed spontaneously or interventionally by device closure prior to the onset of arrhythmias. In this instance it is often necessary to access the left heart, most commonly by performing a transbaffle puncture with a standard Brockenbrough needle or a radiofrequency-powered transseptal needle to gain access to cardiac chambers under echocardiographic and fluoroscopic guidance [25]. Angiographic roadmaps, fluoroscopic and echocardiographic guidance are routinely employed to achieve successful transbaffle perforation and can be further improved with CT- or MRI-fluoroscopy image fusion. Difficulty in accessing chambers is most evident in patients who have undergone extracardiac Fontan procedures for surgical palliation of single ventricle type CHD. Although some extracardiac Fontan baffles are sutured to the atrial wall, there may be a substantial gap between the baffle and atrial wall that is not amenable to transvenous recanalization. Techniques described to overcome this problem include transthoracic percutaneous access to the pulmonary venous atrium under fluoroscopic guidance [26]. Challenges in accessing cardiac chambers for arrhythmia mapping and ablation are not limited to patients who have undergone a Fontan procedure although the single ventricle population are the most likely to require additional pre-ablation procedure planning. Alternative access from the internal jugular vein may be required in patients with heterotaxy associated interrupted inferior vena cava [27]. If these patients have also undergone a bidirectional Glenn, with superior vena cava anastomosed directly to the pulmonary arteries and oversewing of the pulmonary valve, transhepatic access may be necessary [28]. As mentioned above, careful consideration should be given to the potential benefit of preoperative electrophysiology studies and arrhythmia ablation prior to cardiac surgery involving isolation of chambers.
Limitations of size in the pediatric CHD population
Rhythm disturbances associated with complex congenital cardiac anomalies can occur at any age from the fetus to the elderly. A select few fetal cardiac interventions for structural heart disease are currently available while management of fetal arrhythmias is mainly limited to pharmacologic therapy (either delivered maternally or transplacentally) for fetal atrial tachyarrhythmias. Following birth, patient size is the main limiting factor in the risk benefit ratio of transcatheter arrhythmia ablation versus pharmacologic therapy. Risks associated with ablation procedures are heightened in small patients secondary to vascular access issues, the number of catheters placed, the proximity of structures such as the coronary arteries and the intrinsic conduction system to arrhythmia substrate ablation sites. Adequate scar must be created to terminate arrhythmia whilst avoiding damage to surrounding structures. This is further complicated by the increased capacity for cell division of the neonatal myocardium compared with adult myocardium, as evidenced by animal studies, that results in increasing size and significance of scar tissue as the infant grows [29,30]. As expected, the risks of arrhythmia ablation procedures including complete AV block, hemopericardium, femoral artery, femoral vein or iliac venous occlusion are increased in the pediatric population secondary to patient size. Kantoch et al. reported a 10% major complication rate in children less than 2 years of age versus a 0.7% complication rate in children over 2 years of age [31]. The age and weight at which ablation procedures are attempted has decreased steadily over the last few years although 3D mapping is limited to a catheter size of at least 7-Fr diameter. At present ablation procedures are considered reasonable and relatively low risk, if performed in centers with experience, for patients aged 3 years and weight of 15 kg [30,32–35]. There have also been multiple case reports of successful arrhythmia ablation in small infants often with arrhythmias that have been refractory to pharmacologic therapy [36–39] including use of 3D mapping (Figure 1).
Electroanatomic mapping & image integration
3D mapping systems
Standard electrophysiology studies are performed using pacing protocols to elicit arrhythmias. Changes in surface and intracardiac electrograms are analyzed to locate arrhythmia substrate tissue. 3D mapping systems have revolutionized electrophysiology studies reducing procedure time and drastically reducing fluoroscopy time by providing integration of nonfluoroscopic anatomic and catheter position images with electroanatomic data [40–42]. Anatomic shells of each cardiac chamber of interest are created and electrical activation patterns are then superimposed on these shells to create a 3D electroanatomic map (Figures 2B & 1C). For patients with complex CHD, the 3D map may delineate abnormal congenital or postsurgical anatomy thereby increasing likelihood of the procedural success and reducing the risk of complications [43]. Multiple 3D mapping systems are currently available and used with success in the complex CHD population including CARTO®, (Biosense Webster, Diamond Bar, CA, USA) which uses a sensor in the tip of the catheter to identify the catheter location in relation to a magnetic field created by three coils in a locator pad beneath the patient, NavX (St Jude Medical, St Paul, MN, USA) which uses impedance to determine the catheter position and electrical signals during mapping or ablation and EnSite (St Jude Medical, St Paul, MN, USA) which is a noncontact mapping system consisting of a 64-electrode mesh balloon using blood pool electrical signals to map the cardiac chamber endocardium. Propagation maps of conduction in baseline rhythm and after induction of arrhythmia are created based upon the timing of electrical signals. Isthmuses of slow conduction related to ischemia are identified and tissue with low voltage or no electrical activity can be incorporated in the map as scar tissue or surgical patch. This method is highly effective for identification of potential ablation sites in those with complex CHD.
Currently, several centers, including our own, are performing zero fluoroscopy electrophysiology studies and ablation procedures even in the complex pediatric CHD population. Our personal experience is that at least half of our ablation procedures are done with zero to less than 5 min of low-dose fluoroscopy. The resulting radical reduction in radiation exposure is of particular importance in the CHD population. Cardiac ionizing procedures have been shown to be associated with chromosomal damage in children with CHD [44]. Those with complex CHD are likely to require multiple diagnostic and interventional procedures in their lifetime resulting in significant levels of cumulative radiation exposure and an associated estimated median lifetime attributable risk of cancer of approximately 1.6% [45].
Image fusion
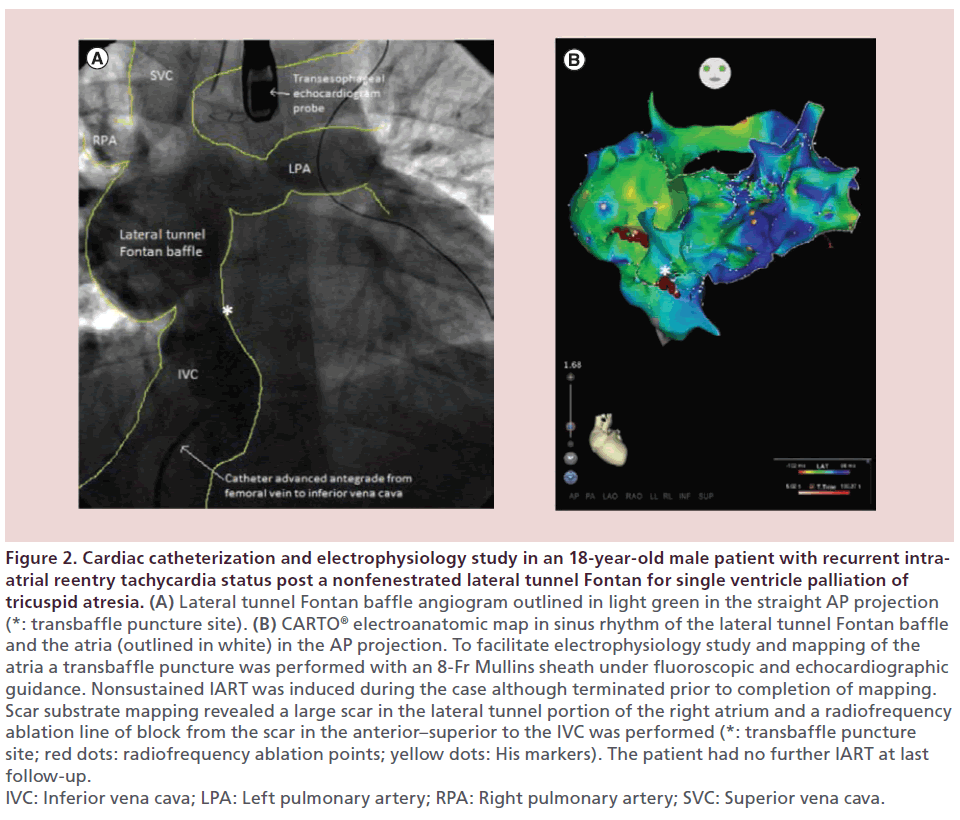
One of the major limitations to procedural success and reaching zero fluoroscopy in the complex CHD population is the difficulty in accessing and mapping abnormal cardiac anatomy and surgically isolated cardiac chambers. Image fusion technology may overcome or at least reduce these limitations in many cases. Adult patients with complex CHD have poor transthoracic echocardiographic windows particularly for evaluation of anterior structures such as the right ventricle. These patients therefore routinely undergo a cardiac MRI prior to interventional procedures to assess anatomy, scar tissue and ventricular function. Integration of magnetic resonance images with the 3D mapping system such as the Cartomerge system (Biosense Webster, Diamond Bar, CA, USA), as shown in Figure 3, is relatively straightforward and can vastly improve operator understanding of the patient’s anatomy, electroanatomic map and catheter position. Data regarding efficacy of Cartomerge compared with conventional 3D mapping technology alone is limited although a reduction in radiation exposure is apparent even in adults with structurally normal hearts [46]. Image fusion of the 3D electroanatomic map can also be performed with intracardiac ultrasound such as the CartoSound system (Biosense Webster, Diamond Bar, CA, USA), and less favorably with radiation exposure associated modalities including CT or fluoroscopy if necessary.
Figure 3: Image fusion of pre-procedure MRI and CARTO® with Cartomerge map of the right atrium in a 26-year- old female patient with atrial tachycardia and history of d-transposition of the great arteries status postatrial switch with Senning baffle in infancy. The cardiac chambers are labelled by color (light blue: right atrium; orange: left atrium and pulmonary veins; purple: morphologic right ventricle; yellow: ascending aorta; red: head and neck vessels; dark blue: morphologic left ventricle; green: main and branch pulmonary arteries) and images are overlayed with variable transparency on the CARTO® map to guide mapping and ablation of arrhythmia substrates. (A) AP projection with full MRI overlay, (B) AP projection with increased transparency of the MRI overlay and (C) PA projection with increased transparency of the MRI overlay.
Advances in catheter & ablation technology
Remote magnetic navigation
Remote controlled magnetic navigation technology such as Niobe II (Stereotaxis Inc, MO, USA), in conjunction with a 3D electroanatomic mapping system has theoretical benefits of improved safety from less traumatic catheter design and improved catheter stability in addition to the possibility of improved operator experience by allowing a single operator to perform procedures remotely at multiple sites [47]. This technology has been shown to be safe and feasible in children and those with CHD resulting in significantly lower radiation exposure than conventional techniques in some reports although procedural success and complications rates are similar to procedures performed by an in person experienced operator [47–49]. The expense of remote magnetic navigation system installation has thus far limited the number of centers where the technology is available and therefore overall experience, particularly in the complex CHD population, is limited.
Specialized catheters & sheaths
In addition to the modifications resulting in decreased size of mapping, recording and ablation catheters and the increase in number electrodes per catheter thus creating multipurpose catheters there have also been tremendous advances in the maneuverability of electrophysiology procedure-specific tools. Deflectable and steerable catheters and sheaths, initially designed for pulmonary vein interventions in adults with structurally normal hearts, have proved to be useful in the complex CHD population allowing access to abnormally positioned cardiac chambers and stable catheter position for ablation in otherwise hard to reach areas.
Radiofrequency ablation
Radiofrequency ablation was introduced as an alternative to the DC catheter ablation technique in the late 1980s with a profoundly improved safety profile. Radiofrequency current results in heating of myocardial tissue to greater than 50°C to create an ablation lesion. Overheating to temperatures greater than 90°C is associated with coagulum formation at the ablation catheter tip resulting in increased electrical impedance, decreased current delivery to the tissue, boiling of the tissues and potential development of a steam pop [50]. Ablation lesion size can be somewhat controlled by setting the radiofrequency power output to achieve a preset temperature, maintaining stable catheter tip position against the desired tissue and monitoring temperature, impedance and intracardiac electrogram effect during lesion creation. Cool-tip radiofrequency ablation catheters allow lesion creation using higher radiofrequency power while avoiding high temperatures at the catheter-tissue interface thus decreasing the risk of coagulum formation, tissue boiling and associated complications. They have also been shown to have increased efficacy compared with conventional radiofrequency catheters [51–53]. Various methods of tip-cooling are possible including irrigated catheters that provide a continuous stream of cold liquid around the catheter tip and closed-loop cooled tip technologies keep the tip from developing char formation. Cool-tip catheters are particularly useful in patients who have undergone Fontan palliation surgery resulting in thick atrial walls secondary to elevated intra-atrial pressure where deep and extensive ablation lesions may be required for successful arrhythmia management [43]. In addition, passive flow in the Fontan circulation limits ambient cooling which can be compensated for with cool-tip catheters. Cool-tip catheters are also used to create deep ablation lesions for management of ventricular tachycardia in patients with significant ventricular hypertrophy associated with CHD such as TOF [51,54]. Irrigated tip catheters with contact force measurement provide feedback regarding tip to tissue contact with improved ablation outcomes in adults with atrial fibrillation and may provide similar increased efficacy in the CHD population [55,56].
Cryoablation
Cryoablation was introduced in the early 2000s with many subsequent reports of successful applications in the pediatric and CHD populations [38]. While data on cryoablation efficacy are limited in the pediatric and CHD population, the technique is frequently employed for ablation of accessory pathways and arrhythmia substrates located in close proximity to the AV node to significantly reduce the risk of heart block. Lesions are created by freezing tissue with the liquid nitrogen cooled catheter tip. In comparison to radiofrequency ablation, cryoablation has multiple benefits that are particularly important in the pediatric and CHD populations [57]. Most importantly, cryoablation allows for reversible cryomapping at a tip temperature of -25°C to -40°C prior to permanent lesion creation with catheter tip temperature of less than -65°C. In addition the catheter tip becomes adhered to the tissue during freezing thus creating a stable tip-tissue position, there is lower risk of damage to surrounding structures and lower risk of intracardiac thrombus. Unfortunately, in comparison to radiofrequency ablation, cryoablation appears to result in a higher rate of arrhythmia recurrence [58].
Outcomes
Compared to those with structurally normal hearts arrhythmia ablation in the CHD population is associated with longer procedure duration, lower acute procedural success rates and higher rate of arrhythmia recurrence [12–14,16,59]. Despite these less than perfect outcomes, transcatheter therapies for arrhythmia in the CHD population have made a tremendous positive impact of in terms of improvements in quality of life [60,61]. For some patients even a few arrhythmia free years following an ablation procedure is a relative success if the negative side effects of pharmacologic therapy have been avoided. In addition, the overall rate of complications, approximately 0.5–2% rate of serious adverse outcomes, is acceptably low despite the aforementioned procedural challenges in the complex CHD population [16,42,62–63].
Arrhythmia ablation acute procedural success rates in the CHD population are dependent on the type of arrhythmia. A single center retrospective review of 83 patients with CHD undergoing transcatheter ablation of AV reciprocating tachycardia found an acute success rate of 80% per procedure with 23% of patients undergoing more than one ablation procedure and a complication rate of 5.5% including one death [59]. Patients with Ebstein’s anomaly had similar acute procedural success rates but were more likely to have arrhythmia recurrence compared with patients with other types of CHD which is in keeping with the expected high rate of multiple accessory pathways in this population. A small study of patients with single ventricle physiology found acute arrhythmia ablation success rates of 73% for intra-atrial tachycardia, 75% for atrial flutter and 100% for focal atrial tachycardia [16]. In adults with CHD the rate of successful ablation of intra-atrial tachycardia has been shown to be lower and the rate of recurrence higher, greater than 40%, in those who have undergone complex atrial surgery [64].
While new arrhythmia substrate formation secondary to abnormal congenital or post-surgical cardiac anatomy may account for some of the arrhythmia recurrence burden, ineffective ablation in the first instance is likely to account for a significant proportion of recurrences. Thick atrial walls that develop following Fontan palliation secondary to elevated intra-atrial pressure may in part be responsible for high recurrence rates in some cases. Without tissue visualization during ablation procedures, relatively standard power and temperature thresholds are used to control lesion size perhaps underestimating the true depth and extent required to successfully terminate arrhythmia in this patient group. Current clinical practice for transcatheter ablation of arrhythmias relies on a combination of fluoroscopy, three dimensional mapping systems, intracardiac electrograms and thermal changes to determine the cardiac anatomy, identify the area of interest, guide and assess creation of a successful ablation lesion. An incomplete ablation lesion with gaps that prevent complete termination of arrhythmia can contribute to the failure of success and risk of recurrence [65]. More accurate objective assessment including visualization of the ablation lesion, particularly one that can be obtained and interpreted as part of the ablation procedure, is likely to be integral to increasing the success rate of ablation procedures especially in patients with complex CHD.
Conclusion
Interventional electrophysiology procedures have a high procedural success rate, low complication rate and provide dramatic improvements in quality of life with avoidance of chronic antiarrhythmic pharmacologic therapy in the complex CHD population. While complex CHD poses technical challenges such as isolation of cardiac chambers, the tremendous advances in techniques and technology seen over the past 30 years have enabled application of transcatheter therapies for patients with every type of congenital defect and multiple arrhythmia substrates for patients of any age with complex CHD. Further innovations in mapping, image integration, ablation catheters and zero fluoroscopy techniques are on track to provide further benefits to the complex CHD population in the coming years.
Future perspective
Development of technology for limiting or eliminating radiation exposure during invasive electrophysiology procedures is an absolute priority. In the current era, many procedures can be performed with zero fluoroscopy and there is a general consensus regarding the need to move away from radiation emitting imaging such as fluoroscopy and computed tomography to reduce cumulative for the complex CHD population in addition to radiation exposure reduction for the electrophysiology operators. Increased utilization of image fusion technology and integration of nonfluoroscopic imaging with the electroanatomic map is likely to further reduce the need for fluoroscopy in complex transcatheter ablation procedures. Transseptal and transbaffle perforation remain challenging without fluoroscopic guidance particularly in the complex CHD population and electrophysiologists must become proficient in use of intracardiac or transesophageal echocardiography and image fusion with the 3D electroanatomic map to guide such procedures.
There is limited data on interventional electrophysiology outcomes in very young patients and in the complex CHD population. Prospective registries capturing date from multiple sites are required to adequately incorporate enough cases and the full spectrum of arrhythmias and CHD. Data from such registries could be compiled to provide more accurate estimation tools for accessory pathway, arrhythmogenic foci and reentrant circuit locations from noninvasive assessment thus improving the likelihood of invasive procedural success with further reduction in complication rates [63].
With further developments of specialized mapping and ablation catheters it will hopefully soon be possible to perform complex electrophysiology procedures on even the smallest infants with integration of 3D mapping data from catheters that are ideally less than 7-Fr diameter. Improvements in ablation catheter energy delivery are also anticipated. In the complex CHD population an energy type and associated delivery system that results in creation of precisely controlled ablation lesions with low risk of damage to the intrinsic AV node, His bundle conduction system and surrounding structures.
Acute procedural success and prevention of long term arrhythmia recurrence are likely to be improved if direct visualization of lesion creation becomes possible rather than the current standard methods that rely on indirect measures of lesion creation such as 3D mapping systems, temperature, catheter impedance and changes in intracardiac electrogram pattern. MRI guided arrhythmia ablation combines avoidance of radiation exposure with excellent delineation of cardiac anatomy and potential for direct assessment of ablation lesion creation in real time. It may be possible to accurately assess whether lines of ablation are complete allowing for ablation of gaps during the procedure [66]. Imaging, catheter, sheath and monitoring technologies to achieve this goal are currently under development with promising results in animal studies and limited preliminary investigations in humans [67–72]. There is an exciting potential for MRI and 3D electroanatomic map guided arrhythmia ablation in the future that will be a next step in the revolution of interventional electrophysiology procedures for the complex CHD population.
Acknowledgements
The authors thank Kohei Sumihara, RCIS, for his assistance with preparation of the figures.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Arrhythmias in complex CHD
• There is an increased burden of arrhythmias in the congenital heart disease (CHD) population secondary to congenital anomalies of the conduction system, physiologic and post-surgical consequences of CHD.
• Arrhythmias in complex CHD are associated with significant morbidity and mortality.
Indications for arrhythmia ablation
• Significant symptoms, syncope or hemodynamic instability particularly when there has been failure or intolerance of medical therapy.
• Ablation procedures are considered reasonable and relatively low risk, if performed in centers with experience, for patients aged 3–5 years and weight of 15–20 kg.
• Ablation procedures can be successfully performed in small infants with modified techniques if the arrhythmia is refractory to pharmacologic therapy.
Challenges of electrophysiology interventions in the CHD population
• Limitations of patient size can be overcome with single catheter mapping and ablation techniques.
• Access to cardiac chambers isolated by abnormal congenital anatomy or post cardiac surgery can be safely achieved with transseptal or transbaffle puncture guided by multimodality imaging techniques.
Electroanatomic mapping
• Advances in 3D electroanatomic mapping have been particularly beneficial for the complex CHD population.
• Image fusion of 3D electroanatomic map with echo, MRI and/or fluoroscopic images reduces procedure time and radiation exposure.
• Zero fluoroscopy procedures are possible even in patients with complex CHD.
Ablation technologies
• Cool-tip catheters allow for deeper radiofrequency ablation lesion creation that may be necessary for thick atrial walls in patients who have undergone a Mustard, Senning or Fontan procedure or in a hypertrophied ventricle.
• Cryoablation is useful to reduce the risk of AV block when the anticipated ablation site is in close proximity to the intrinsic conduction system.
Future perspective
• Further advances in interventional electrophysiology must continue to reduce and if possible exclude fluoroscopy for the complex CHD population with cumulative lifetime radiation exposure risks.
• MRI-guided ablation is on the horizon with exciting potential benefits for the complex CHD population.
References
Papers of special note have been highlighted as: • of interest
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 39(12), 1890–1900 (2002).
- Greutmann M, Tobler D, Kovacs AH et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit. Heart Dis. doi: 10.1111/chd.12201 (2014) (Epub ahead of print).
- Walsh EP. Sudden death in adult congenital heart disease: risk stratification in 2014. Heart Rhythm 11(10), 1735–1742 (2014).
- Cuypers JA, Opic P, Menting ME et al. The unnatural history of an atrial septal defect: longitudinal 35 year follow up after surgical closure at young age. Heart 99(18), 1346–1352 (2013).
- Khairy P, Aboulhosn J, Gurvitz MZ et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation 122(9), 868–875 (2010).
- Aanhaanen WT, Moorman AF, Christoffels VM. Origin and development of the atrioventricular myocardial lineage: insight into the development of accessory pathways. Birth Defects Res. Part A Clin. Mol. Teratol. 91(6), 565–577 (2011).
- Kurosawa H, Kawada N. The conduction system in heterotaxy. World J. Pediatr. Congenit. Heart Surg. 2(2), 275–277 (2011).
- Epstein MR, Saul JP, Weindling SN, Triedman JK, Walsh EP. Atrioventricular reciprocating tachycardia involving twin atrioventricular nodes in patients with complex congenital heart disease. J. Cardiovasc. Electrophysiol. 12(6), 671–679 (2001).
- Bae EJ, Noh CI, Choi JY et al. Twin AV node and induced supraventricular tachycardia in Fontan palliation patients. Pacing Clin. Electrophysiol. 28(2), 126–134 (2005).
- Bharati S, Lev M. The course of the conduction system in single ventricle with inverted (L-) loop and inverted (L-) transposition. Circulation 51(4), 723–730 (1975).
- Anderson RH, Becker AE, Arnold R, Wilkinson JL. The conducting tissues in congenitally corrected transposition. Circulation 50(5), 911–923 (1974).
- Zachariah JP, Walsh EP, Triedman JK et al. Multiple accessory pathways in the young: the impact of structural heart disease. Am. Heart J. 165(1), 87–92 (2013).
- Mandapati R, Walsh EP, Triedman JK. Pericaval and periannular intra-atrial reentrant tachycardias in patients with congenital heart disease. J. Cardiovasc. Electrophysiol. 14(2), 119–125 (2003).
- Verma A, Marrouche NF, Seshadri N et al. Importance of ablating all potential right atrial flutter circuits in postcardiac surgery patients. J. Am. Coll. Cardiol. 44(2), 409–414 (2004).
- Greene AE, Skinner JR, Dubin AM, Collins KK, Van Hare GF. The electrophysiology of atrioventricular nodal re-entry tachycardia following the Mustard or Senning procedure and its radiofrequency ablation. Cardiol. Young 15(6), 611–616 (2005).
- De Groot NM, Atary JZ, Blom NA, Schalij MJ. Long-term outcome after ablative therapy of postoperative atrial tachyarrhythmia in patients with Congenit. Heart Dis. and characteristics of atrial tachyarrhythmia recurrences. Circ. Arrhythm. Electrophysiol. 3(2), 148–154 (2010).
- Carlson AM, Turek JW, Law IH, Von Bergen NH. Pseudo-preexcitation is prevalent among patients with repaired complex congenital heart disease. Pediatr. Cardiol. 36(1), 8–13 (2014).
- Bar-Cohen Y, Khairy P, Morwood J, Alexander ME, Cecchin F, Berul CI. Inaccuracy of Wolff-Parkinson-white accessory pathway localization algorithms in children and patients with congenital heart defects. J. Cardiovasc. Electrophysiol. 17(7), 712–716 (2006).
- Collins KK, Love BA, Walsh EP, Saul JP, Epstein MR, Triedman JK. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am. J. Cardiol. 86(9), 969–974 (2000).
- Sherwin ED, Triedman JK, Walsh EP. Update on interventional electrophysiology in congenital heart disease: evolving solutions for complex hearts. Circ. Arrhythm. Electrophysiol. 6(5), 1032–1040 (2013).
- Mah DY, Alexander ME, Cecchin F, Walsh EP, Triedman JK. The electroanatomic mechanisms of atrial tachycardia in patients with tetralogy of Fallot and double outlet right ventricle. J. Cardiovasc. Electrophysiol. 22(9), 1013–1017 (2011).
- Zeppenfeld K, Schalij MJ, Bartelings MM et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation 116(20), 2241–2252 (2007).
- Esch JJ, Triedman JK, Cecchin F, Alexander ME, Walsh EP. Radiofrequency-assisted transseptal perforation for electrophysiology procedures in children and adults with repaired congenital heart disease. Pacing Clin. Electrophysiol. 36(5), 607–611 (2013).
- Graham LN, Melton IC, Macdonald S, Crozier IG. Value of CT localization of the fossa ovalis prior to transseptal left heart catheterization for left atrial ablation. Europace 9(6), 417–423 (2007).
- Correa R, Walsh EP, Alexander ME et al. Transbaffle mapping and ablation for atrial tachycardias after mustard, senning, or Fontan operations. J. Am. Heart Assoc. 2(5), e000325 (2013).
- Nehgme RA, Carboni MP, Care J, Murphy JD. Transthoracic percutaneous access for electroanatomic mapping and catheter ablation of atrial tachycardia in patients with a lateral tunnel Fontan. Heart Rhythm 3(1), 37–43 (2006).
- Lim HE, Pak HN, Tse HF, Lau CP, Hwang C, Kim YH. Catheter ablation of atrial fibrillation via superior approach in patients with interruption of the inferior vena cava. Heart Rhythm 6(2), 174–179 (2009).
- Singh SM, Neuzil P, Skoka J et al. Percutaneous transhepatic venous access for catheter ablation procedures in patients with interruption of the inferior vena cava. Circ. Arrhythm. Electrophysiol. 4(2), 235–241 (2011).
- Khairy P, Guerra PG, Rivard L et al. Enlargement of catheter ablation lesions in infant hearts with cryothermal versus radiofrequency energy: an animal study. Circ. Arrhythm. Electrophysiol. 4(2), 211–217 (2011).
- Blaufox AD, Paul T, Saul JP. Radiofrequency catheter ablation in small children: relationship of complications to application dose. Pacing Clin. Electrophysiol. 27(2), 224–229 (2004).
- Kantoch MJ, Gulamhusein SS, Sanatani S. Short- and long-term outcomes in children undergoing radiofrequency catheter ablation before their second birthday. Can. J. Cardiol. 27(4), e523–e529 (2011).
- Aiyagari R, Saarel EV, Etheridge SP, Bradley DJ, Dick M 2nd, Fischbach PS. Radiofrequency ablation for supraventricular tachycardia in children < or =15 kg is safe and effective. Pediatr. Cardiol. 26(5), 622–626 (2005).
- Svintsova LI, Popov SV, Kovalev IA. Radiofrequency ablation of drug-refractory arrhythmias in small children younger than 1 year of age: single-center experience. Pediatr. Cardiol. 34(6), 1321–1329 (2013).
- An HS, Choi EY, Kwon BS et al. Radiofrequency catheter ablation for supraventricular tachycardia: a comparison study of children aged 0–4 and 5–9 years. Pacing Clin. Electrophysiol. 36(12), 1488–1494 (2013).
- Turner CJ, Lau KC, Sholler GF. Outcomes of interventional electrophysiology in children under 2 years of age. Cardiol. Young 22(5), 499–506 (2012).
- Kornyei L, Konta L, Szatmari A, Rosenthal E. Successful ablation of an incessant ventricular tachycardia in a 3.5 kg infant. Europace 16(2), 283 (2014).
- Femenia F, Sarquella-Brugada G, Brugada J. Single-catheter radiofrequency ablation of a permanent junctional reciprocating tachycardia in a premature neonate. Cardiol. Young 22(5), 606–609 (2012).
- Dean PN, Skeete A, Moak JP, Berul CI. Cryoablation and angiographic evidence of a concealed right atrial appendage to right ventricle accessory pathway in an infant. Congenit. Heart Dis. 8(6), E183–E187 (2013).
- Costello JP, He D, Greene EA, Berul CI, Moak JP, Nath DS. Radiofrequency catheter ablation of intractable ventricular tachycardia in an infant following arterial switch operation. Congenit. Heart Dis. 9(2), E46–E50 (2014).
- Kwong W, Neilson AL, Chiu CC et al. The effect of NavX on fluoroscopy times in pediatric catheter ablation. J. Interv. Cardiac Electrophysiol. 33(1), 123–126 (2012).
- Pass RH, Gates GG, Gellis LA, Nappo L, Ceresnak SR. Reducing patient radiation exposure during paediatric SVT ablations: use of CARTO® 3 in concert with “ALARA” principles profoundly lowers total dose. Cardiol. Young doi:10.1017/S1047951114001474 (2014) (Epub ahead of print).
- Papagiannis J, Avramidis D, Alexopoulos C, Kirvassilis Radiofrequency ablation of accessory pathways in children and Congenit. Heart Dis. patients: impact of a nonfluoroscopic navigation system. Pacing Clin. Electrophysiol. 34(10), 1288–1396 (2011).
- Triedman JK, Delucca JM, Alexander ME, Berul CI, Cecchin F, Walsh EP. Prospective trial of electroanatomically guided, irrigated catheter ablation of atrial tachycardia in patients with congenital heart disease. Heart Rhythm 2(7), 700–705 (2005).
- Andreassi MG, Ait-Ali L, Botto N, Manfredi S, Mottola G, Picano E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur. Heart J. 27(22), 2703–2708 (2006).
- Johnson JN, Hornik CP, Li JS et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation 130(2), 161–167 (2014).
- Caponi D, Corleto A, Scaglione M et al. Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome?: a randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace 12(8), 1098–1104 (2010).
- Akca F, Bauernfeind T, Witsenburg M et al. Acute and long-term outcomes of catheter ablation using remote magnetic navigation in patients with congenital heart disease. Am. J. Cardiol. 110(3), 409–414 (2012).
- Roudijk RW, Gujic M, Suman-Horduna I, Marchese P, Ernst Catheter ablation in children and young adults: is there an additional benefit from remote magnetic navigation? Neth. Heart J. 21(6), 296–303 (2013).
- Suman-Horduna I, Babu-Narayan SV, Ueda A et al. Magnetic navigation in adults with atrial isomerism (heterotaxy syndrome) and supraventricular arrhythmias. Europace 15(6), 877–885 (2013).
- Haines DE, Verow AF. Observations on electrode-tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation 82(3), 1034–1038 (1990).
- Nabar A, Rodriguez LM, Timmermans C, Wellens HJ. Use of a saline-irrigated tip catheter for ablation of ventricular tachycardia resistant to conventional radiofrequency ablation: early experience. J. Cardiovasc. Electrophysiol. 12(2), 153–161 (2001).
- Okishige K, Aonuma K, Yamauchi Y et al. Clinical study of the efficacy of a cooled-tip catheter ablation system for common atrial flutter. Circ. J. 68(1), 73–76 (2004).
- Jais P, Shah DC, Haissaguerre M et al. Prospective randomized comparison of irrigated-tip versus conventional-tip catheters for ablation of common flutter. Circulation 101(7), 772–776 (2000).
- Morwood JG, Triedman JK, Berul CI et al. Radiofrequency catheter ablation of ventricular tachycardia in children and young adults with congenital heart disease. Heart Rhythm 1(3), 301–308 (2004).
- Sigmund E, Puererfellner H, Derndorfer M et al. Optimizing radiofrequency ablation of paroxysmal and persistent atrial fibrillation by direct catheter force measurement – a case-matched comparison in 198 patients. Pacing Clin. Electrophysiol. 38(2), 201–208 (2014).
- Natale A, Reddy VY, Monir G et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J. Am. Coll. Cardiol. 64(7), 647–656 (2014).
- Lapage MJ, Reed JH, Collins KK et al. Safety and results of cryoablation in patients <5 years old and/or <15 kilograms. Am. J. Cardiol. 108(4), 565–571 (2011).
- Collins KK, Rhee EK, Kirsh JA et al. Cryoablation of accessory pathways in the coronary sinus in young patients: a multicenter study from the Pediatric and Congenital Electrophysiology Society’s Working Group on Cryoablation. J. Cardiovasc. Electrophysiol. 18(6), 592–597 (2007).
- Chetaille P, Walsh EP, Triedman JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart Rhythm 1(2), 168–173 (2004).
- Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation 94(7), 1585–1591 (1996).
- Bathina MN, Mickelsen S, Brooks C, Jaramillo J, Hepton T, Kusumoto FM. Radiofrequency catheter ablation versus medical therapy for initial treatment of supraventricular tachycardia and its impact on quality of life and healthcare costs. Am. J. Cardiol. 82(5), 589–593 (1998).
- Ueda A, Suman-Horduna I, Mantziari L et al. Contemporary outcomes of supraventricular tachycardia ablation in congenital heart disease: a single-center experience in 116 patients. Circ. Arrhythm. Electrophysiol. 6(3), 606–613 (2013).
- Triedman JK, Pfeiffer P, Berman A et al. COMPASS: a novel risk-adjustment model for catheter ablation in pediatric and Congenital Heart Disease patients. Congenit. Heart Dis. 8(5), 393–405 (2013).
- Yap SC, Harris L, Silversides CK, Downar E, Chauhan VS. Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J. Am. Coll. Cardiol. 56(19), 1589–1596 (2010).
- Melby SJ, Lee AM, Zierer A et al. Atrial fibrillation propagates through gaps in ablation lines: implications for ablative treatment of atrial fibrillation. Heart Rhythm 5(9), 1296–1301 (2008).
- Reddy VY, Schmidt EJ, Holmvang G, Fung M. Arrhythmia recurrence after atrial fibrillation ablation: can magnetic resonance imaging identify gaps in atrial ablation lines? J. Cardiovasc. Electrophysiol. 19(4), 434–437 (2008).
- Dickfeld T, Kato R, Zviman M et al. Characterization of radiofrequency ablation lesions with gadolinium-enhanced cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 47(2), 370–378 (2006).
- Ranjan R, Kholmovski EG, Blauer J et al. Identification and acute targeting of gaps in atrial ablation lesion sets using a real-time magnetic resonance imaging system. Circ. Arrhythm. Electrophysiol. 5(6), 1130–1135 (2012).
- Vergara GR, Vijayakumar S, Kholmovski EG et al. Real-time magnetic resonance imaging-guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Heart Rhythm 8(2), 295–303 (2011).
- Bisbal F, Guiu E, Cabanas-Grandio P et al. CMR-guided approach to localize and ablate gaps in repeat AF ablation procedure. JACC Cardiovasc. imaging 7(7), 653–663 (2014).
- Piorkowski C, Grothoff M, Gaspar T et al. Cavotricuspid isthmus ablation guided by real-time magnetic resonance imaging. Circ. Arrhythm. Electrophysiol. 6(1), e7–e10 (2013).
- Grothoff M, Piorkowski C, Eitel C et al. MR imaging-guided electrophysiological ablation studies in humans with passive catheter tracking: initial results. Radiology 271(3), 695–702 (2014).
• Review of postoperative atrial tachyarrhythmia transcatheter ablation acute procedural success rates and long-term recurrence rate showing that recurrence of arrhythmia originates from different locations.
• Single center review of 118 cases (96% success rate) of transbaffle puncture for attempted arrhythmia ablation following repair of complex coronary heart disease with surgical isolation of cardiac chambers.
• Retrospective review of 193 ablation procedures showing that older age and history of complex atrial surgery reduce likelihood of procedural success.
• Describes the initial experience of MRI-guided arrhythmia ablation with limited acute procedural success in a series of ten adult patients with atrial flutter in structurally normal hearts.