Research Article - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 3
Towards stratified treatment of rheumatoid arthritis
- Corresponding Author:
- Nathalie Luurssen-Masurel
1Department of Rheumatology
Erasmus MC, Dr. Molewaterplein 40 3015 GD Rotterdam, Netherlands
E-mail: n.luurssen-masurel@erasmusmc.nl
Abstract
Background: To compare the clinical efficacy of different initial treatment strategies in autoantibodynegative rheumatoid arthritis(RA) patients. Methods and findings: Data of the tREACH trial, a stratified single-blinded randomized clinical trial with a treat-to-target strategy, were used. For this analysis, we selected all autoantibodynegative RA patients, defined as fulfillment of 2010-criteria and absence of both rheumatoid factor and anti-citrullinated protein antibody, within the intermediate probability stratum. We compared the following initial treatment strategies in our autoantibody-negative RA population: 25mg methotrexate(iMTX) per week, 400mg hydroxychloroquine(iHCQ) daily or 15mg glucocorticoids(iGCs) orally in a 10-week tapering scheme without any DMARDs. Primary outcome was the proportion of patients with active disease, defined as a disease activity score(DAS)≥2.4, after 3 months of treatment. Secondary outcomes were DAS and functional ability(HAQ) over time using a linear mixed model(LMM), in which we respectively corrected for baseline DAS and HAQ. 116 patients were included and started with iMTX(n=44), iHCQ(n=35) or iGCs(n=37). After 3 months 34%, 34% and 76% of patients respectively treated with iMTX, iHCQ and iGCs had an active disease(p<.0005 for iHCQ and iMTX versus iGCs). Our corrected LMM showed no significant difference in DAS and HAQ over time between the different initial treatment strategies. Conclusions: Initial GCs without csDMARDs are also not indicated for autoantibody-negative RA patients. However, iHCQ and iMTX show similar (early) treatment responses in this subgroup of patients, which suggests that initial treatment can be stratified for autoantibody-negative and autoantibody-positive RA, but validation is needed.
Keywords
rheumatoid arthritis • autoantigens and autoantibodies • DMARDs • NSAIDs • biological therapies
Key messages
What is already known about this subject?
• Literature suggests that rheumatoid arthritis patients without autoantibodies may be treated with less intensive therapy with similar outcomes and possibly less adverse events. What does this study add?
• This is the first study that investigated the clinical efficacy of different initial treatment strategies in newly diagnosed autoantibody-negative rheumatoid arthritis patients. How might this impact on clinical practice or future developments?
• An initial oral glucocorticoid-tapering scheme of 10 weeks without any csDMARDs is also not indicated for autoantibody-negative RA patients.
• Initial hydroxychloroquine and methotrexate show similar (early) treatment responses in autoantibody-negative rheumatoid arthritis patients, suggesting that initial treatment can be stratified for autoantibody-negative and autoantibody-positive RA, but validation is needed.
Introduction
Clinical and radiological outcomes in Rheumatoid Arthritis (RA) have improved enormously in the last decades, due to early detection of the disease, early initiation of ‘intensive’ therapy and a treat-to-target approach [1]. Early diagnosis and treatment has led to a greater diversity in the clinical phenotype of RA, which led to an increase of RA patients without autoantibodies, also known as autoantibody-negative RA [2].
The presence of autoantibodies, Anti-Citrullinated Protein Antibody (ACPA) and/or Rheumatoid Factor (RF), is associated with worse treatment response and outcome [3]. Even current treatment recommendations advise to consider more intensive therapy for autoantibody-positive RA compared to autoantibodynegative RA, when they have an inadequate response on their first-line DMARD strategy. However, the question remains whether these different disease subsets can be treated differently from the start, with less intensive firstline therapy for autoantibody-negative RA.
Previous literature already showed that autoantibodynegative RA patients had a better treatment response than autoantibody-positive RA patients when given similar therapies [3]. Although autoantibody-negative RA patients show a better response to similar therapy, the impact of the disease on patients’ lives is comparable to autoantibody-positive RA patients [4,5]. Unfortunately, there are – to our knowledge - no studies comparing different initial treatment strategies in autoantibodynegative RA patients.
Current guidelines, recommend starting with Methotrexate (MTX). The disadvantage of MTX are side effects such as nausea, vomiting and diarrhea [6]. Trials show that MTX is discontinued in 7-16% of patients due to side effects [7,8]. Aforementioned problems may be circumvented with less toxic therapy.
To summarise, literature suggests that autoantibodynegative RA may be treated with less intensive therapy with similar outcomes and possibly less adverse events. However, data about the comparison of initial treatment strategies in autoantibody-negative RA are lacking [5].
Therefore, we compared the clinical efficacy of three different initial treatment strategies in newly diagnosed autoantibody-negative RA patients, according to 2010 criteria [1].
Methods
Patients
For this study, we used the data of the treatment in the Rotterdam Early Arthritis Cohort (tREACH) trial. The tREACH trial was a multicenter, stratified single-blinded trial [8]. In this trial patients were stratified into three probability strata, namely low, intermediate and high, according to their likelihood of progressing to persistent arthritis, which was based upon the prediction model of Visser et al. [9]. For further details we would like to refer to the publications of Claessen et al. [10] and Visser et al. [9] Medical ethics committees at each participating center approved the tREACH study protocol, and all patients gave written informed consent before inclusion (MEC-2006-252). Reporting of this study will follow the CONSORT guidelines.
Within the original tREACH trial 495 (78%) patients fulfilled the 2010 criteria for RA and of those patients 164 (33%) had autoantibody-negative RA and thus were selected for our study.(1) Fulfilment of the 2010 criteria was mainly due to joint involvement, swollen or tender, in >10 joints (n=152, 93%). A small proportion of patients fulfilled the 2010 criteria based upon erosions typical for RA (n=12, 7%).
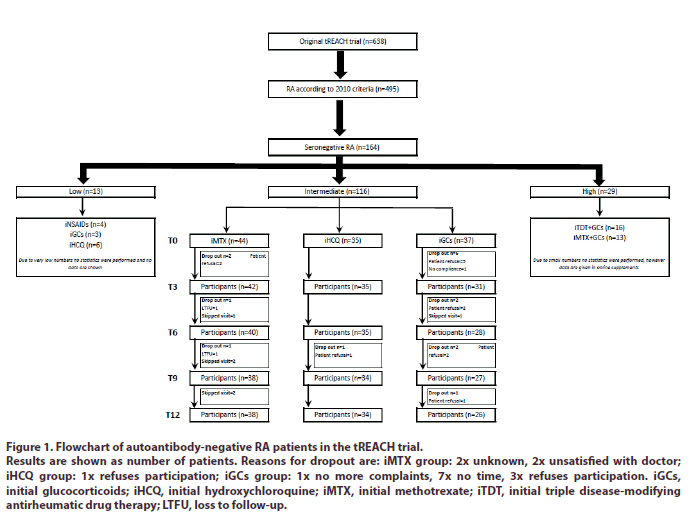
The selected autoantibody-negative RA patients had the following distribution over the original tREACH probability strata: 13 (8%) low, 116 (71%) intermediate and 29 (18%) high (Figure 1). Due to very low numbers, we excluded all autoantibody-negative RA patients within the low probability stratum from our analysis. Data of the autoantibody-negative RA patients in the high probability stratum on the other hand are shown in the online supplements. However, no conclusions can be drawn from them due to small numbers and their different initial treatment strategies. Hence, we will mainly focus on the autoantibody-negative RA patients within the intermediate probability stratum.
Figure 1: Flowchart of autoantibody-negative RA patients in the tREACH trial.
Results are shown as number of patients. Reasons for dropout are: iMTX group: 2x unknown, 2x unsatisfied with doctor; iHCQ group: 1x refuses participation; iGCs group: 1x no more complaints, 7x no time, 3x refuses participation. iGCs, initial glucocorticoids; iHCQ, initial hydroxychloroquine; iMTX, initial methotrexate; iTDT, initial triple disease-modifying antirheumatic drug therapy; LTFU, loss to follow-up.
Intervention
Patients in the intermediate stratum received one of the following initial treatment strategies: Methotrexate (iMTX) 25mg per week (n=50), Hydroxychloroquine (iHCQ) 400mg daily (n=40) or Glucocorticoids (iGCs) in a 10-week oral tapering scheme starting with 15mg per day (n=41) without any csDMARDs in the first 3 months. Folic acid (10mg/week) was given to all patients using MTX. Osteoporosis prophylaxis (risedronate 35mg/week and calcium/vitamin D combination 500/400 mg/IU/day) was given during the first 3 months in the iGCs group. According to the protocol none of the patients receiving iMTX or iHCQ had a GC bridging scheme.
Design
The tREACH trial had a treat-to-target approach. Treatment decisions were based upon the original Disease Activity Score (DAS) threshold for low disease activity (DAS<2.4).(11) If the RA was still active (DAS≥2.4), treatment was intensified in the following order:
• triple DMARD therapy, consisting of MTX, sulfasalazine and HCQ
• MTX + etanercept (50mg/week, subcutaneously)
• MTX + adalimumab (40mg/2 weeks, subcutaneously) and
• MTX + abatacept (500-1000mg/4 weeks, intravenously, weight dependent)
Treatment intensifications could occur at each 3 monthly visit and in case of very active disease, based on the rheumatologists’ insight, an earlier visit could be planned and if necessary treatment could be intensified. Medication was tapered if DAS was <1.6 at two consecutive visits with a 3-monthly interval. The order of tapering steps was
• biological agent
• sulfasalazine
• MTX and
• HCQ
All medications were gradually tapered, except for HCQ, which was stopped immediately. Patients receiving iGCs who had a low disease activity after 10 weeks were in Drug-Free Remission (DFR) from that moment.
Outcomes and assessment
Visits occurred every 3 months and at each visit the DAS, medication usage and self-reported questionnaires were collected, except for hand/foot radiographs, which were examined at baseline, at 6 months and 1 year.
Our primary outcome was the proportional difference in patients with an active disease (DAS≥2.4) after 3 months of treatment. Our secondary outcomes are:
• Disease activity after 3 months
• Disease activity over time
• Functional ability over time
• Medication usage over time
• Biological usage after 12 months
• Proportion of patients in DMARD-Free Remission (DFR) after 12 months of therapy and
• Proportion of patients with radiological progression during the first year of follow-up.
Disease states were determined with DAS and its thresholds (moderate to high disease activity: DAS≥2.4; low disease activity: 1.6≥ DAS <2.4 and remission: DAS <1.6) [11]. Functional ability was measured with the Health Assessment Questionnaire (HAQ) [12]. Higher HAQ scores represent poorer function. Radiological progression was measured with the modified Total Sharp Score (mTSS) [13]. Radiographs were read chronically by two out of three qualified assessors, who were blinded for the patients’ treatment allocation [14]. Weighted kappa for the total mTSS between assessors was 0.79 with 99% agreement. Proportion of patients with radiological progression was defined as a change in mTSS >0.5 and >0.7 (the smallest detectable change). This is in agreement with guidelines for presentation of radiological results in clinical trials [15]. Drug-Free Remission (DFR) was defined as having a DAS<1.6 without DMARD therapy [8].
Safety monitoring and toxicity
Safety monitoring was done according to Dutch guidelines and consisted of laboratory tests at fixed intervals [16-18]. In case of (serious) adverse events the dosage was lowered or medication was stopped, according to the insight of the treating physician [10]. If patients showed gastrointestinal complaints, MTX could be given subcutaneously. If for safety reasons MTX had to be discontinued, it was substituted with leflunomide (20 mg/day) [10].
Statistical analysis
The sample-size calculation of the original tREACH study was based upon the area under the curve (AUC) of the HAQ, using data from the BeSt study, where mean AUC HAQ of combination therapy and monotherapy respectively was 7.7 (SD 5.5) and 10.5 (SD 7.4). A target sample size of 270 patients per probability stratum (90 patients per arm) was needed to detect the mentioned difference in AUC HAQ with a power of 80% and a two-sided α=.05 [19].
We are well aware that this is a post-hoc analysis and, therefore, statistical comparisons between initial treatment strategies are only performed in autoantibodynegative RA patients within the intermediate probability stratum. Based on the numbers per treatment arm and our primary outcome (proportional difference in active disease after 3 months of therapy) and considering previous published 3-months data of the tREACH we are able to detect a 30% difference with a power of 80% and a two-sided α=.05 [20].
Clinical efficacy was calculated in an Intention-To- Treat (ITT) analysis. In an ITT analyses patients are analysed in the groups to which they were randomized, regardless of whether they received or adhered to the allocated intervention. Missing values at each timepoint were imputed. Radiological progression was extraor interpolated if mTSS was missing at 12 months. A χ2 test was used to measure the proportional difference in active disease after 3 months of treatment and a student’s t-test was used to compare DAS at 3 months. For the DAS and HAQ over time we will use a Linear Mixed Model (LMM) with an unstructured covariance matrix, where time, treatment and baseline DAS and HAQ are respectively the covariates.
All other statistical comparisons were made by student’s t-test, γ2 test, or Wilcoxon rank-sum test, when appropriate. Means were presented for normally distributed data and medians for non-normally distributed data. Statistical analyses were performed using STATA V.15.1. A p-value <.05 was considered statistically significant.
Results
Patients
The baseline characteristics of the 116 autoantibodynegative RA patients, within the intermediate probability stratum, per initial treatment strategy are given in Table 1. Patients were mostly female (70%) with a median symptom duration of 134 days (IQR: 95-205) (Table 1). Patients started with iMTX (n=44), iHCQ (n=35) or iGCs (n=37) (Figure 1).
Table 1. Baseline characteristics and clinical response for each induction therapy group.
| Characteristics | iMTX (n=44) | iHCQ (n=35) | iGCs (n=37) |
|---|---|---|---|
| Demographic | |||
| Age (years), mean (SD) | 56 (14) | 55 (14) | 53 (14) |
| Sex, female, n (%) | 33 (75) | 22 (63) | 26 (70) |
| Disease characteristics | |||
| Symptom duration (days), median (IQR) | 137 (85-209) | 140 (101-213) | 124 (94-192) |
| Disease activity | |||
| DAS, mean (SD) | 3.51 (0.92) | 3.00 (0.85) | 3.57 (0.94) |
| TJC44, median (IQR) | 13 (8-19) | 12 (6-14) | 13 (8-17) |
| SJC44, median (IQR) | 9 (6-13) | 6 (2-10) | 8 (4-15) |
| General health, median (IQR)a | 53 (40-70) | 44 (28-59) | 49 (25-68) |
| ESR in mm/h, median (IQR) | 14 (7-29) | 11 (6-23) | 20 (12-40) |
| CRP in mg/L, median (IQR) | 5 (4-23) | 5 (2-12) | 6 (2-18) |
| Radiographs (hand/foot) | |||
| mTSS (0-488), median (IQR) | 0 (0-2) | 0 (0-1) | 0 (0-1s) |
| Erosion score (0-280), median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| JSN score (0-168), median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-0) |
| Erosive disease, n (%)b | 1 (2) | 0 (0) | 0 (0) |
| Functional ability | |||
| HAQ, mean (SD) | 1.22 (0.51) | 0.91 (0.62) | 1.02 (0.68) |
aGeneral health is measured with a Visual Analogue Scale from 0 to 100 mm.
bErosive disease is defined as having an erosion score >1 in three separate joints.
CRP: C-reactive protein; DAS: Disease Activity Score; ESR: Erythrocyte Sedimentation Rate; HAQ: Health Assessment Questionnaire; iGCs: initial glucocorticoids; iHCQ: initial hydroxychloroquine; iMTX: initial methotrexate; iTDT: initial triple disease-modifying antirheumatic drug therapy; JSN: Joint Space Narrowing; mTSS: modified Total Sharp Score; RA: Rheumatoid Arthritis; SASP: Sulfasalazine; SJC44: Swollen Joint Count (44 joints); TJC44: Tender Joint Count (44 joints)
Inadequate responders after 3 months
Respectively 34%, 34% and 76% initially treated with MTX, HCQ and GCs had an active disease (DAS ≥2.4) at 3 months, and thus needed a treatment intensification (p<.0005 for iHCQ and iMTX versus iGCs). No significant difference between iHCQ and iMTX was seen.
Disease activity (over time)
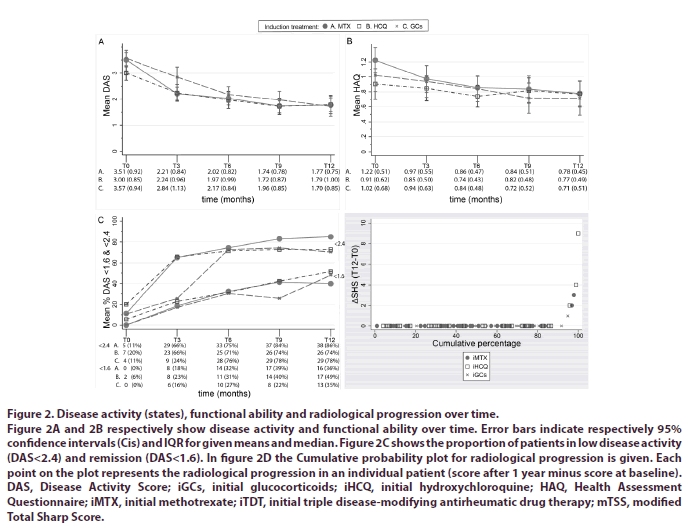
DAS at 3 months was significantly higher for patients receiving iGCs (DAS=2.84) compared to iHCQ (DAS=2.24, p<.05) and iMTX (DAS=2.21, p<.005). Again, no differences were seen between iHCQ and iMTX. Our corrected LMM showed no significant difference in DAS over time between patients receiving iHCQ compared to iMTX. DAS over time was also not significantly different between patients treated with iGCs compared to the other initial treatment strategies (Figure 2A).
Figure 2: Disease activity (states), functional ability and radiological progression over time.
Figure 2A and 2B respectively show disease activity and functional ability over time. Error bars indicate respectively 95% confidence intervals (Cis) and IQR for given means and median. Figure 2C shows the proportion of patients in low disease activity (DAS<2.4) and remission (DAS<1.6). In figure 2D the Cumulative probability plot for radiological progression is given. Each point on the plot represents the radiological progression in an individual patient (score after 1 year minus score at baseline). DAS, Disease Activity Score; iGCs, initial glucocorticoids; iHCQ, initial hydroxychloroquine; HAQ, Health Assessment Questionnaire; iMTX, initial methotrexate; iTDT, initial triple disease-modifying antirheumatic drug therapy; mTSS, modified Total Sharp Score.
Functional ability
For functional ability over time the adjusted difference in our LMM between patients receiving iMTX compared to iHCQ was .01 (95% CI -.12 to .14) and compared to iGCs .05 (95% CI -.09 to .19). The adjusted difference between patients receiving iHCQ and iGCs was -.06 (95%CI -.19 to .07) (Figure 2B).
Radiological progression
The median increase (interquartile range, IQR) in mTSS was respectively 0.8 (0-2.5), 0 (0-1) and 0 (0-1) for patients receiving iMTX, iHCQ and iGCs. Respectively 5%, 9% and 3% of patients treated with iMTX, iHCQ or iGCs had radiological progression, defined as an increase in mTSS of >0.7 (Table 2) [21]. The cumulative probability plot of radiological progression for the 3 initial treatment strategies were superimposable (Figure 2C and 2D).
Table 2. Clinical response for each induction therapy group after 12 months.
| Clinical response | iMTX (n=38) | iHCQ (n=34) | iGCs (n=26) |
|---|---|---|---|
| Disease activity | |||
| DAS, mean (SD) | 1.77 (0.75) | 1.79 (1.00) | 1.70 (0.85) |
| TJC44, median (IQR) | 2 (0-9) | 1 (0-8) | 0 (0-4) |
| SJC44, median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) |
| General health, median (IQR)a | 33 (19-50) | 30 (11-55) | 30 (8-48) |
| ESR in mm/h, median (IQR) | 7 (4-15) | 9 (5-13) | 9 (5-23) |
| CRP in mg/L, median (IQR) | 2 (1-5) | 3 (1-6) | 3 (1-6) |
| ∆DAS (T12-T0), mean (SD) | -1.74 (1.11) | -1.22 (0.95) | -1.87 (1.14) |
| Radiographs (hand/foot) | |||
| mTSS (0-488), median (IQR) | 0.8 (0-2.5) | 0 (0-1) | 0 (0-1) |
| Erosion score (0-280), median (IQR) | 0 (0-0) | 0 (0-0.5) | 0 (0-0) |
| JSN score (0-168), median (IQR) | 0 (0-1.3) | 0 (0-0.5) | 0 (0-0.3) |
| ∆mTSS (T12-T0), median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| Patients with progression >0.5, n (%) | 2 (5) | 3 (9) | 1 (3) |
| Patients with progression >0.7, n (%) | 2 (5) | 3 (9) | 1 (3) |
| Erosive disease, n (%)b | 2 (5) | 0 (0) | 0 (0) |
| Functional ability | |||
| HAQ, mean (SD) | 0.78 (0.45) | 0.77 (0.49) | 0.71 (0.51) |
| ∆HAQ (T12-T0), mean (SD) | -0.44 (0.60) | -0.13 (0.75) | -0.31 (0.56) |
aGeneral health is measured with a Visual Analogue Scale from 0 to 100 mm.
bErosive disease, defined as having an erosion score >1 in three separate joints.
CRP: C-Reactive Protein; DAS: Disease Activity Score; ESR: Erythrocyte Sedimentation Rate; iGCs: initial glucocorticoids; iHCQ: initial hydroxychloroquine; iMTX: initial methotrexate; HAQ: Health Assessment Questionnaire; iTDT: initial Triple Disease-modifying antirheumatic drug Therapy; JSN: Joint Space Narrowing; mTSS: modified Total Sharp Score; RA: Rheumatoid Arthritis; SASP: sulfasalazine; SJC44: Swollen Joint Count (44 joints); TJC44: Tender Joint Count (44 joints)
Medication
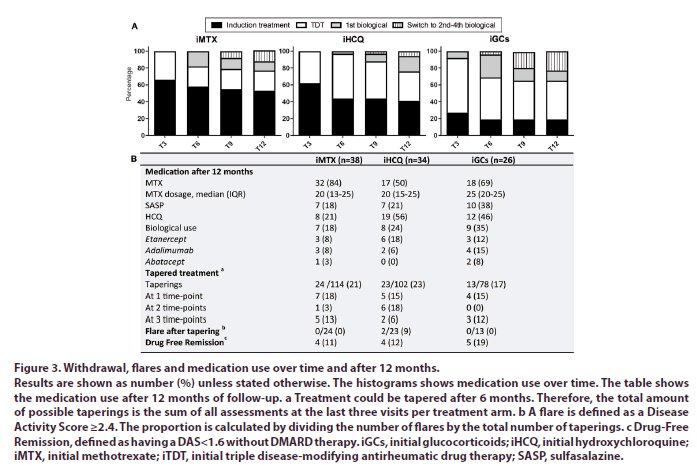
After 1 year patients receiving iGCs needed more treatment adjustments to reach low disease activity compared to iMTX (p<.05) (Figure 3A). Furthermore, 18%, 24% and 35% of patients treated with respectively iMTX, iHCQ or iGCs were using biologicals after 1 year of follow-up. Because of sustained remission 34% (n=13), 38% (n=13) and 27% (n=7) of the patients respectively receiving iMTX, iHCQ and iGCs were able to taper their treatment. Of the patients who were able to taper their medication 31% (n=4), 31% (n=4) and 71% (n=5) respectively receiving iMTX, iHCQ and iGCs were in drug-free remission (Figure 3B).
Figure 3: Withdrawal, flares and medication use over time and after 12 months.
Results are shown as number (%) unless stated otherwise. The histograms shows medication use over time. The table shows the medication use after 12 months of follow-up. a Treatment could be tapered after 6 months. Therefore, the total amount of possible taperings is the sum of all assessments at the last three visits per treatment arm. b A flare is defined as a Disease Activity Score ≥2.4. The proportion is calculated by dividing the number of flares by the total number of taperings. c Drug-Free
Remission, defined as having a DAS<1.6 without DMARD therapy. iGCs, initial glucocorticoids; iHCQ, initial hydroxychloroquine; iMTX, initial methotrexate; iTDT, initial triple disease-modifying antirheumatic drug therapy; SASP, sulfasalazine.
Adverse events (AEs)
A total of 265 AEs were self-reported. The most commonly reported AEs among all patients are gastrointestinal complaints (65/265, 25%), fatigue (31/265, 12%) and skin problems (28/265, 11%). Overall, no differences were seen in the number of (serious) AEs and proportion of patients with ≥1 AE(s) between the initial treatment strategies (Table 3). However, the tolerance of the initial treatment is in our opinion best reflected by the reported (serious) AEs at 3 months, which were slightly in favour of iHCQ, but non-significant (Table 3).
Table 3. Number (%) of patients with (serious) adverse events and treatment alterations due to side effects.
| iMTX (n=44) | iHCQ (n=35) | iGCs (n=37) | |
|---|---|---|---|
| Adverse events (AEs) | |||
| In first year | |||
| Serious AE(s)a | 3 (7) | 2 (6) | 0 (0) |
| Patients with ≥1 AE(s) | 13 (30) | 10 (29) | 6 (16) |
| No of AEs per patient, median (IQR) | 3 (1-6) | 3 (0-4) | 3 (1-5) |
| At 3 months | |||
| Serious AE(s) | 1 (2) | 0 (0) | (0) |
| Patients with ≥1 AE(s) | 30 (68) | 19 (54) | 21 (57) |
| No of AEs per patient, median (IQR) | 1 (0-3) | 1 (0-2) | 1 (0-2) |
| Medication changes due to AEs in first year | |||
| Switch to MTX SC | 9 (20) | 4 (11) | 6 (16) |
| Lowering MTX dosage <=20 mg/wk | 26 (59) | 10 (29) | 12 (32) |
| Stop MTX | 3 (7) | 1 (3) | 1 (3) |
| Stop SASP | 1 (2) | 1 (3) | 0 (0) |
| Stop HCQ | 4 (9) | 1 (3) | 5 (14) |
| Stop biological agents | 2 (5) | 0 (0) | 1 (3) |
| Observed AEs in first year | |||
| Bone marrow depressionb | 8 (18) | 2 (6) | 7 (19) |
| High creatinine | 0 (0) | 1 (3) | 1 (3) |
| Elevated liver enzymes | 5 (11) | 2 (6) | 2 (5) |
| Hyperglycemia | 0 (0) | 0 (0) | 0 (0) |
Results are shown as number (%) unless stated otherwise.
aSerious AEs per treatment are respectively: arm (MTX) 1x pneumonia with IC and reanimation 1x sun allergy and 1x cardiac arrhythmia with pulmonary edema ; arm (HCQ) 1x CTS with 2 times OK and 1x ICD wire had to be removed and rearranged.
bBone marrow depression is defined as an anaemia, thrombocytopenia, or leucopenia with respectively a hemoglobin level, platelet count and white blood cells count below the lower limit of the normal range. High creatinine, raised liver enzymes and hyperglycemia are defined as having respectively a creatinine, liver transaminases and glucose level above the upper limit of the normal range.
iGCs: initial glucocorticoids; iHCQ: initial hydroxychloroquine; iMTX: initial methotrexate; SASP: sulfasalazine; SC: subcutaneous
If we look at treatment adjustments due to AEs, than most adjustments occurred in the first 3 months, namely 28 of the 87 (32%) adjustments. All treatment adjustments due to adverse events at 3 months were based upon intolerance for MTX, while no adjustments due to AEs occurred in the iHCQ and iGCs groups.
Discussion
Ideally, treatment of RA is tailored to the individual patient. Rheumatologists strive for quick attainment of low disease activity, but they also want to prevent overtreatment. More intensive treatment is associated with more side effects. Therefore, we compared the clinical efficacy of three different initial treatment strategies in newly diagnosed autoantibody-negative RA patients in the intermediate probability stratum of the tREACH trial. Patients treated with iGCs had a more active disease at 3 months compared to patients receiving either iMTX or iHCQ. iMTX and iHCQ on the other hand showed similar treatment responses after 3 months and over time. Patients receiving iGCs also needed more treatment intensifications to reach low disease activity compared to the other initial treatment strategies. Again, no differences were seen between iMTX and iHCQ. Functional ability and radiological progression did not differ between the different initial treatment strategies. Treatment adjustments due to adverse events in the first 3 months only occurred in the iMTX group. To summarize, iGCs are also not indicated for this subgroup of patients. iHCQ and iMTX on the other hand show similar (early) treatment responses and outcomes.
This is the first study that evaluated different initial treatment strategies in autoantibody-negative RA patients. Although, Choi et al. already showed that autoantibody-negative RA had a better clinical response with similar therapy compared to autoantibody-positive RA, they did not compare different initial treatment strategies within autoantibody-negative RA.(3) We have shown that iGCs are also not indicated for this subgroup of patients. iHCQ and iMTX on the other hand show similar (early) treatment responses and outcomes, which suggests that stratified treatment of RA based upon autoantibody status is possible, but validation is needed.
The possible advantage of initial HCQ over MTX is that it is better tolerated [22]. In our study for example we observed more treatment adjustments due to adverse events and also more self-reported adverse events in the first 3 months of follow-up in the initial MTX group, but these differences were non-significant.
A concern on the other hand might be the effect of HCQ on radiological progression. A recent review showed that HCQ halts radiological progression, but this was less compared to the other csDMARDs [23]. However, most of the included studies for this review were published before 2000 and thus included RA patients according to the 1987 criteria. Moreover, results were not stratified for autoantibody status, which is a strong predictor for radiological progression. In addition, a treat-to-target approach will halt radiological progression [24,25].
If autoantibody-negative RA can be treated with less intensive therapy, one would think that treatment could also be (completely) tapered more often. In our study 13% of autoantibody-negative RA patients were in Drug-Free Remission (DFR) after 1 year of follow-up, which is similar to DFR rates in other RA trials [26-28]. Therefore, autoantibody-negative RA is not a selflimiting disease that passes naturally.
Our study had certain limitations. First, we are well aware that this a post-hoc analyses and that results should be interpreted carefully. To minimize the chance of false positive tests, we prespecified our outcomes and corrected for baseline imbalances.
Secondly, there were also autoantibody-negative RA patients within the low and high probability stratum. Due to very low numbers and different initial treatment strategies, we excluded these patients from our analysis. However, in the online supplements we have shown the data of the autoantibody-negative RA patients in the high probability stratum. After 12 months of treatment, DAS, functional ability and radiological progression did not seem to differ between these different initial treatment strategies. This is in accordance with our other results. This suggests that even in autoantibody-negative patients with a high DAS and/or erosions at baseline less intensive treatment is sufficient. However, more data are needed to validate this hypothesis.
Finally, the total number of dropouts was higher in the iGC group compared to the other initial treatment strategies. Our results show that iGCs are less effective after 3 months of therapy compared to the other initial treatment strategies, which might have caused the higher dropout ratio. If that were the case, this would only lead to even stronger findings for this study.
The strengths on the other hand are the extensive clinical, radiological and adverse events data. Furthermore, we had diverse outcomes of which the results were complementary to each other. This strengthens our conclusions.
In addition, our study contributes to the idea that stratified treatment of RA based upon autoantibody status is possible. The conceptual basis of these findings were already confirmed in current guidelines and previous literature [1,4,29]. For example, current treatment recommendations advise to consider more intensive therapy for autoantibody-positive RA compared to autoantibody-negative RA, when they fail on their first-line DMARD strategy [1,4,29]. However, we propose that autoantibody status should already be taken into account at time of diagnosis instead of after 3 months of treatment [1,30].
Conclusion
Initial glucocorticoids without csDMARDs are also not indicated for autoantibody-negative RA patients. However, initial hydroxychloroquine and methotrexate show similar (early) treatment responses in this subgroup of patients, which suggest that initial treatment can be stratified for autoantibody-negative and autoantibodypositive RA, but validation is needed.
Ethics approval
Medical Ethics committee of the Erasmus MC [MEC-2006-252].
Consent for publication
All patients gave written informed consent before inclusion (MEC-2006-252).
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Competing interest
The authors have declared no conflicts of interest.
Funding
The tREACH trial was supported by an unrestricted grant from Pfizer bv. [WI229707]. Pfizer had no involvement in the study design; in collection, analysis and interpretation of data; writing of the report; and decision to submit for publication. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication. Data management was sponsored by the Dutch Arthritis Society (16- 3-101).
Authors contributions
All authors contributed to the conception or design of the trial; or the acquisition, analysis, or interpretation of data for the work; drafting or revision of the manuscript; and final approval of the manuscript for publication. All authors contributed to refinement of the article and approved the final manuscript.
Acknowledgements
We especially thank the participating patients in the tREACH trial for their willingness to contribute to the study and for their cooperation. We thank all rheumatologists from the following participating centers: Erasmus MC, Rotterdam; Maasstad ziekenhuis, Rotterdam; Sint Fransiscus Gasthuis & Vlietland, Rotterdam & Schiedam; Albert Schweitzer ziekenhuis, Dordrecht; Admiraal de Ruyter ziekenhuis, Goes en Vlissingen; and Zorgsaam ziekenhuis, Terneuzen. We also thank all study-nurses, laboratory personnel, co-investigators and others who were involved with the tREACH study. This work has been previously presented at the Dutch Society for Rheumatology (NVR) conference and published as a conference abstract at the EULAR.
References
- Smolen JS, Landewe RBM, Bijlsma JWJ et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 79(6), 685–699 (2020).
- Boeters DM, Mangnus L, Ajeganova S et al. The prevalence of ACPA is lower in rheumatoid arthritis patients with an older age of onset but the composition of the ACPA response appears identical. Arthritis. Res. Ther. 19(1), 115 (2017).
- Choi ST, Lee KH. Clinical management of seronegative and seropositive rheumatoid arthritis: A comparative study. PLoS One. 13(4), e0195550 (2018).
- Boer AC, Boonen A, van der Helm van Mil AHM. Is Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis Still a More Severe Disease Than Anti-Citrullinated Protein Antibody-Negative Rheumatoid Arthritis? A Longitudinal Cohort Study in Rheumatoid Arthritis Patients Diagnosed From 2000 Onward. Arthritis. Care. Res (Hoboken). 70(7), 987–996 (2018).
- Pratt AG, Isaacs JD. Seronegative rheumatoid arthritis: pathogenetic and therapeutic aspects. Best. Pract. Res. Clin. Rheumatol. 28(4), 651–659 (2014).
- https://www.lareb.nl/nl/databank/Result?formGroup=&atc=L04AX03&ref=fk
- Choy EH, Smith CM, Farewell V et al. Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Ann. Rheum. Dis. 67(5), 656–663 (2008).
- de Jong PH, Hazes JM, Han HK et al. Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann. Rheum. Dis. 73(7), 1331–1339 (2014).
- Visser H, le Cessie S, Vos K et al. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis. Rheum. 46(2), 357–365 (2002).
- Claessen SJ, Hazes JM, Huisman MA et al. Use of risk stratification to target therapies in patients with recent onset arthritis; design of a prospective randomized multicenter controlled trial. BMC. Musculoskelet. Disord. 10(1), 71 (2009).
- van der Heijde DM, van't Hof M, van Riel PL et al. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J. Rheumatol. 20(3), 579–581 (1993).
- Siegert CE, Vleming LJ, Vandenbroucke JP et al. Measurement of disability in Dutch rheumatoid arthritis patients. Clin. Rheumatol. 3(3), 305–309 (1984).
- van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J. Rheumatol. 27(1), 261–263 (2000).
- van Tuyl LH, van der Heijde D, Knol DL et al. Chronological reading of radiographs in rheumatoid arthritis increases efficiency and does not lead to bias. Ann. Rheum. Dis. 73(2), 391–395 (2014).
- van der Heijde D, Simon L, Smolen J et al. How to report radiographic data in randomized clinical trials in rheumatoid arthritis: guidelines from a roundtable discussion. Arthritis. Rheum. 47(2), 215–218 (2002).
- https://www.nvr.nl/wp-content/uploads/2018/09/NVR-Medicijnen-MTX-richtlijn-2009-update-2011.pdf
- https://www.nvr.nl/wp-content/uploads/2018/09/NVR-Medicijnen-richtlijn-Sulfasalazine-2002.pdf
- https://www.nvr.nl/wp-content/uploads/2018/09/NVR-Medicijnen-Update_Biologicals_richtlijn-23-6-2014.pdf
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis. Rheum. 58(2 Suppl), S126-S135 (2008).
- de Jong PH, Hazes JM, Barendregt PJ et al. Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: first results of the tREACH trial. Ann. Rheum. Dis. 72(1), 72–78 (2013).
- van der Heijde D, van der Helm-van Mil AH, Aletaha D et al. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Ann. Rheum. Dis. 72(4), 479–481 (2013).
- Tom S. Kelley and Firestein’s Textbook of Rheumatology, 2-volume Set, 10th Edition (2017) Pp: 964.
- Rempenault C, Combe B, Barnetche T et al. Clinical and Structural Efficacy of Hydroxychloroquine in Rheumatoid Arthritis: A Systematic Review. Arthritis. Care. Res (Hoboken). (2019).
- Grigor C, Capell H, Stirling A et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 364(9430), 263–269 (2004).
- Verstappen SM, Jacobs JW, van der Veen MJ et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann. Rheum. Dis. 66(11), 1443–1449 (2007).
- Heimans L, Wevers-de Boer KV, Visser K et al. A two-step treatment strategy trial in patients with early arthritis aimed at achieving remission: the IMPROVED study. Ann. Rheum. Dis. 73(7), 1356–1361(2014) .
- Akdemir G, Heimans L, Bergstra SA et al. Clinical and radiological outcomes of 5-year drug-free remission-steered treatment in patients with early arthritis: IMPROVED study. Ann. Rheum. Dis. 77(1), 111–118 (2018).
- Klarenbeek NB, van der Kooij SM, Guler-Yuksel M et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann. Rheum. Dis. 70(2), 315–319 (2011).
- de Moel EC, Derksen V, Trouw LA et al. In RA, becoming seronegative over the first year of treatment does not translate to better chances of drug-free remission. Ann. Rheum. Dis. (2018).
- Luurssen-Masurel N, Weel AEAM, Hazes JMW et al. SAT0078 Which cdmard strategy is most effective in newly diagnosed seronegative rheumatoid arthritispatients; post-hoc analysis of the treach study. Ann. Rheum. Dis. 77(Suppl 2), 901 (2018).