Case Report - Interventional Cardiology (2022) Volume 14, Issue 4
Successful treatment of Swan-Ganz catheter induced pulmonary artery pseudoaneurysm with a detachable coil
- Corresponding Author:
- Yusuf Alibrahim
Department of Medical Imaging,
University of Toronto,
Toronto,
Canada,
E-mail: yusuf.alibrahim@mail.utoronto.ca
Received date: 13-Jun-2022, Manuscript No. FMIC-22-66452; Editor assigned: 15-Jun-2022, PreQC No. FMIC-22-66452 (PQ); Reviewed date: 04-Jul-2022, QC No. FMIC-22-66452; Revised date: 11-Jul-2022, Manuscript No. FMIC-22-66452 (R); Published date: 18-Jul-2022, DOI: 10.37532/1755- 5310.2022.14(4).543
Abstract
This case report describes the use of an endovascular technique for treating a complication of Swan-Ganz catheterization. Despite being rare, injury to the pulmonary artery is a complication of Swan-Ganz catheterization. Arterial catheterization can lead to mechanical perforation and hence creation of an extravascular sac, or pseudoaneurysm. With the advancements of endovascular procedures, a variety of treatment options currently exist in the treatment of pulmonary arteries pseudoaneurysm. In this case presentation, we demonstrate the value of detachable coils in the treatment of Swan- Ganz catheter induced pulmonary artery pseudoaneurysms.
Keywords
Pulmonary artery pseudoaneurysm • Detachable coils • Swan-Ganz catheterization • Transcatheter coil insertion
Introduction
Pulmonary Artery Pseudoaneurysm (PAP) is a rare but life-threatening complication of Swan-Ganz catheterization. Prompt recognition and management of this complication is crucial for improving patient outcome. Endovascular coiling is an established way for treating PAP. The Swan-Ganz catheter was advanced and pressure measurements were obtained. However, the patient developed gross volume hemoptysis highly concerning for pulmonary artery rupture. Herein, we discuss the value of using detachable coils in the treatment of Swan-Ganz catheter induced PAP, particularly in difficult-to-reach areas of the pulmonary vasculature.
Case Presentation
An 88-year-old woman presents to the outpatient cardiology clinic with moderate aortic stenosis, query low flow and low gradient severe aortic stenosis. Furthermore, she has symptoms of worsening fatigue and dyspnea on exertion. Her medical history is significant for heart failure with left ventricular ejection fraction of 40%-45%, hypertension, and aortic stenosis, in addition to coronary artery disease, Parkinson’s disease, and a previous transient ischemic attack. The patient was referred for repeat coronary angiography with left and right heart catheterization as part of the workup for consideration of transcatheter aortic valve implantation.
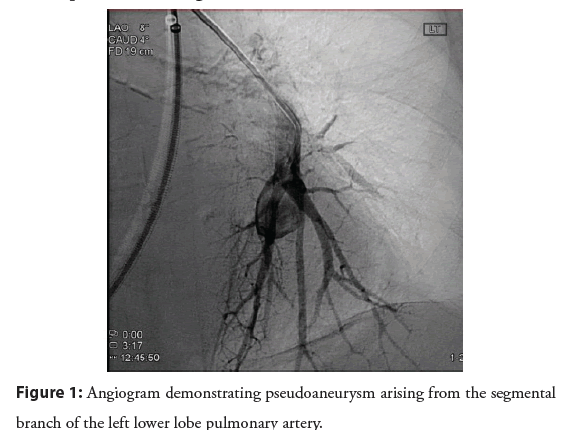
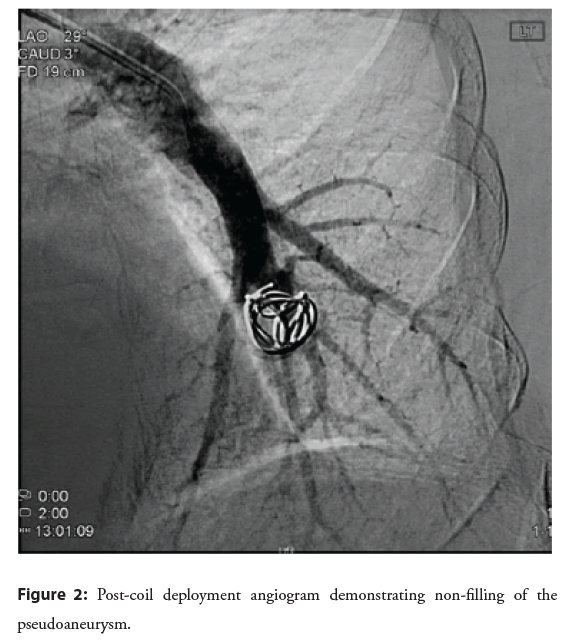
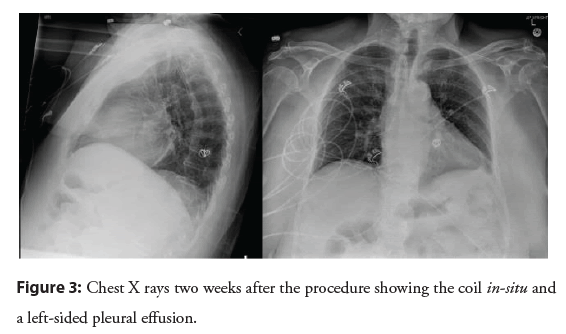
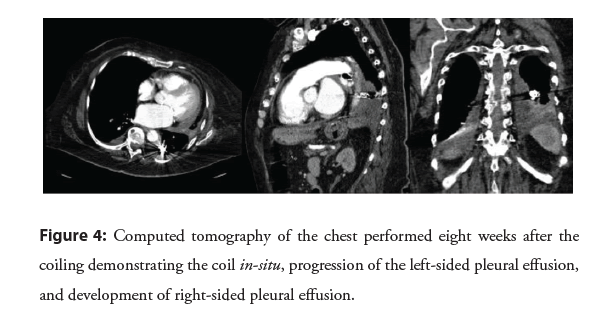
After the patient arrived in the cardiac catheterization lab, right and left heart catheterization were started with femoral vein and artery access using a 7 French and a 6 French sheath, respectively under ultrasound guidance. The Swan-Ganz catheter was advanced and pressure measurements were obtained. However, the patient developed gross volume hemoptysis highly concerning for pulmonary artery rupture. A code blue was called and critical care medicine arrived to assess the patient and assist with airway management. Interventional Radiology (IR) was also emergently consulted and the Swan-Ganz catheter balloon was kept inflated until IR arrival. After IR arrival to the catheterization lab, a guide wire was inserted. Nevertheless, it was difficult to advance the guidewire with the Swan-Ganz balloon inflated, and hence it was deflated. Through the right femoral vein antegrade sheath, a curved pigtail catheter was used to cannulate into the left pulmonary artery. Leftsided pulmonary angiograms were performed which demonstrated a pseudoaneurysm arising from a segmental branch from a left lower lobe pulmonary artery with no evidence of ongoing pulmonary artery bleeding. The patient was hemodynamically stable with a stable airway and no further hemoptysis. She was, therefore, taken emergently to the interventional radiology angiography suite for treatment. In the interventional radiology department, over a guidewire, the pigtail catheter was removed from the femoral vein including the 7-French vascular sheath. This was followed by the insertion of a long 7-French guiding sheath for support with its distal tip placed in the main pulmonary artery. Using a 5-French simple curve catheter, we cannulated down to the left lower lobe pulmonary artery. Multiple pulmonary angiograms were performed to delineate the anatomy and identify the pulmonary artery pseudoaneurysm arising from the segmental branch of the left lower lobe (Figure 1). We were able to eventually cannulate the 5-French simple curve catheter directly into the aneurysmal sac. Next, we deployed a 15 mm × 20 cm detachable coil (Interlock-35 Fibered IDC Occlusion System from Boston Scientific) into the aneurysmal sac. Post-coil embolization angiograms demonstrated excellent angiographic results with no further opacification of the aneurysm (Figure 2). As a central line was requested, we then exchanged the 7-French long guiding sheath for 9.5 French standard length vascular sheath. After right femoral angiograms were performed through the retrograde right femoral arterial sheath, hemostases was achieved by the deployment of an AngioSeal closure device. The patient tolerated the procedure well. There were no immediate postprocedural complications with good outcome on follow up after 2 weeks in Figure 3 and after 8 weeks of the procedure in Figures 4 and 5.
Figure 1: Angiogram demonstrating pseudoaneurysm arising from the segmental branch of the left lower lobe pulmonary artery.
Figure 2: Post-coil deployment angiogram demonstrating non-filling of the pseudoaneurysm.
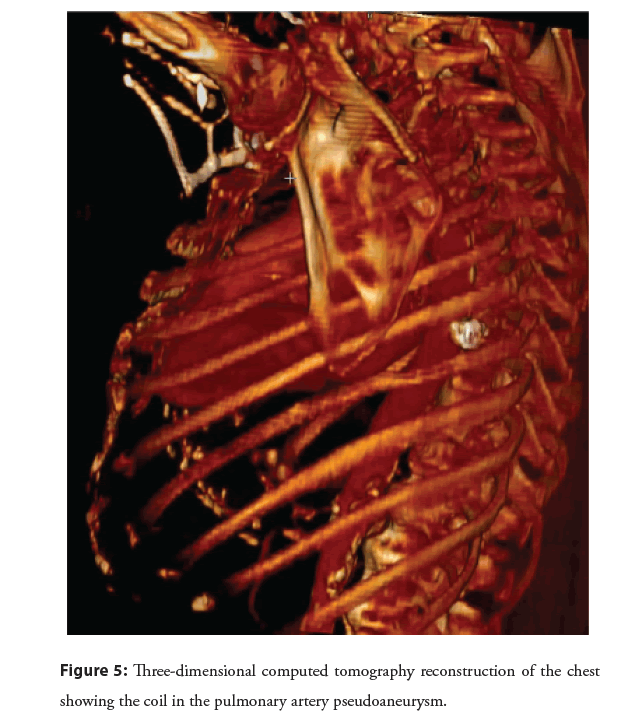
Figure 5: Three-dimensional computed tomography reconstruction of the chest showing the coil in the pulmonary artery pseudoaneurysm.
Results and Discussion
Swan-Ganz catheterization (also called right heart catheterization or pulmonary artery catheterization) was introduced in clinical use by H. J. C. Swan and William Ganz in 1970. With its flowdirected and balloon-tip, it can easily be advanced into the pulmonary artery. Despite the ease of reach to the pulmonary artery, the vital structures nearby risk several complications. Lifethreatening complications induced by Swan-Ganz catheterization are estimated to occur at a frequency of 2% to 17% [1]. The risk of pseudoaneurysm formation due to traumatic injury of the pulmonary artery can occur in 0.66% to 2% of right heart catheterizations, and this carries the risk of hemorrhage, with a reported mortality as high as 50% due to asphyxia and aspiration from intrapulmonary hemorrhage [2].
Clinically, symptoms of Pulmonary Artery Pseudoaneurysm (PAP) depend on the type and the cause. A mispositioned Swan-Ganz catheter can cause an intravascular PAP with symptoms of massive hemoptysis [3]. Extravascular PAP due to penetrating trauma can result in shock, chest pain, dyspnea, and desaturation, in addition to hemoptysis [4]. The risk factors for PAP include pulmonary hypertension, chronic steroid use, age above 60 years old, female sex, and systemic anticoagulation, in addition to cardiac decompression or manipulation during surgery and balloon hyperinflation [5].
The gold standard for diagnosis of pulmonary artery pseudoaneurysm is digital subtraction angiography. CT scans with a single-slice technique can also diagnose the aneurysm but exact anatomic localization would not be possible due to poor resolution in the z axis with this technique. On the other hand, multislice CT has improved spatial resolutions that can be used to visualize the exact anatomy of the aneurysm and analyze the bleeding lesion to determine the feeder artery [6].
Although pulmonary artery pseudoaneurysm is a rare condition, treatment is necessary when there is hemoptysis and risk of rupture. Furthermore, the treatment of PAP depends on its etiology. Symptomatic or high-risk PAP can be treated endovascularly or by surgical techniques. The advancements in interventional radiology makes it an increasingly valuable alternative to surgery and sometimes the only option in non-surgical candidates. Surgical options include aneurysmorrhaphy, lobectomy, aneurysmectomy, and pneumonectomy. However, these options carry a high risk of morbidity and mortality. Endovascular techniques are relatively quick, highly effective, and can sometimes be safe even in anticoagulated patients [7,8]. Transcatheter coil insertion is the most common endovascular treatment for PAP [9]. The advantage of which compared to surgical resection include preserving the pulmonary arteries distal to the aneurysm and, therefore, sparing lung function.
In our case, we opted for treating the PAP with a detachable coil as opposed to a pushable coil. This is because detachable coils have a hook at the end that can be used to pull back the coil if deployment is not optimal, thereby allowing for fine control of the coil.
Conclusion
In conclusion, prompt recognition of pulmonary artery pseudoaneurysms after Swan-Ganz catheterization is imperative to improve patient outcome. If perforation of the pulmonary artery is suspected, keeping the Swan-Ganz catheter balloon inflated is prudent until the situation is assessed and diagnosis is confirmed with angiography. Furthermore, detachable coils have shown promise in the treatment of many aneurysms. In this brief case, the use of detachable coils for Swan-Ganz catheter-induced pulmonary artery pseudoaneurysms is encouraged, particularly in more challenging situations, as it allows for more control during coil deployment.
References
- Labrunie E, Levy C, Paugam C, et al. Pulmonary artery pseudoaneurysm caused by a Swan-Ganz catheter and treated by embolization. Ann Radiol (Paris). 36(4): 310-4 (1993).
[Google Scholar] [PubMed]
- Poplausky MR, Rozenblit G, Rundback JH, et al. Swan-Ganz catheter-induced pulmonary artery pseudoaneurysm formation: Three case reports and a review of the literature. Chest. 120(6): 2105-11 (200).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Guillaume B, Vendrell A, Stefanovic X, et al. Acquired pulmonary artery pseudoaneurysms: A pictorial review. Br J Radiol. 90(1073): 20160783 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Lafita V, Borge MA, Demos TC. Pulmonary artery pseudoaneurysm: Etiology, presentation, diagnosis, and treatment. Semin Intervent Radiol. 24(1): 119-123 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Burrel M, Real MI, Barrufet M, et al. Pulmonary artery pseudoaneurysm after Swan-Ganz catheter placement: Embolization with vascular plugs. J Vasc Interv Radiol. 21(4): 577-81 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Kierse R, Jensen U, Helmberger H, et al. Value of multislice CT in the diagnosis of pulmonary artery pseudoaneurysm from Swan-Ganz catheter placement. J Vasc Interv Radiol. 15(10): 1133-1137 (2004).
[CrossRef] [Google Scholar] [PubMed]
- Lerardi AM, Xhepa G, Musazzi AM, et al. Endovascular treatment of a pulmonary artery pseudoaneurysm caused by Swan-Ganz catheter deployment in an anticoagulated patient. BJR Case Reports. 1(3): 20150064 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Rudziński PN, Demkow M, Michałowska I, et al. Endovascular treatment of PA pseudoaneurysm caused by Swan-Ganz catheter. Postepy Kardiol Interwencyjnej. 10(1): 66-70 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Park A, Cwikiel W. Endovascular treatment of a pulmonary artery pseudoaneurysm with a stent graft: Report of two cases. Acta Radiol. 48(1): 45-7 (2007).
[CrossRef] [Google Scholar] [PubMed]