Case Report - Journal of Interventional Nephrology (2024) Volume 7, Issue 1
Sodium-Glucose Cotransporter 2 Inhibitor Luseogliflozin Treatment Reduces the Declining Speed of Kidney Function in Patients with CKD Stage G5 Diabetic Nephropathy
- Corresponding Author:
- Takafumi Ito
Department of Internal Medicine, Division of Nephrology,Teikyo University Chiba Medical Center, Chiba, Japan
E-mail: terawaki@med.teikyo-u.ac.jp
Received: 30-Jan-2024, Manuscript No. OAIN-24-126282; Editor assigned: 01-Feb-2024, PreQC No. OAIN-24-126282 (PQ); Reviewed: 15-Feb-2024, QC No. OAIN-24-126282; Revised: 22-Feb-2024, Manuscript No. OAIN-24-126282 (R); Published: 29-Feb-2024, DOI: 10.47532/oain.2024.7(1).205-208
Abstract
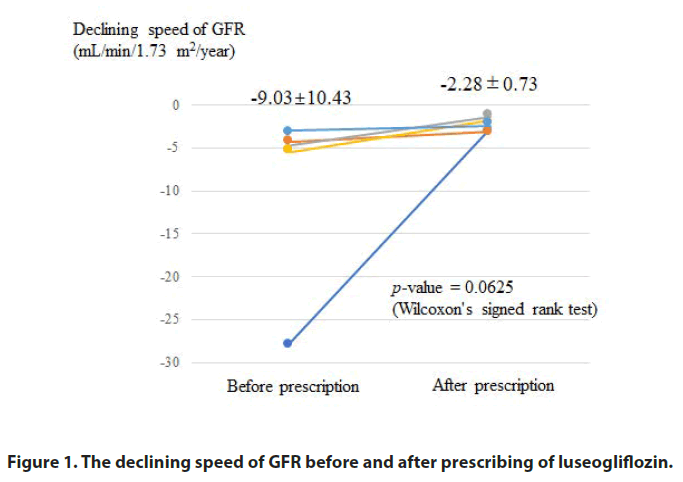
Here we report our experience with respect to the prescription of the Sodium-Glucose Cotransporter 2 inhibitor (SGLT2i) luseogliflozin in patients with CKD stage G5 Diabetic Nephropathy (DN). A total of 5 patients with DN with severe kidney dysfunction estimated Glomerular Filtration Rate (GFR); 6.18-14.82 mL/min/1.73 m2 prescribed luseogliflozin (2.5 mg/day) in addition to the already prescribed drug. After that, the declining speed of GFR (mL/min/1.73 m2/year) decreased in all cases, from -9.03 ± 10.43 to -2.28 ± 0.73, and the degree of reduction was correlated with the declining speed of GFR before treatment. All patients were able to select their preferred modality of Kidney Replacement Therapy (KRT) through a shared decision-making process before starting KRT. We believe that the use of the SGLT2i is beneficial for patients with DN even in CKD stage G5.
Keywords
SGLT2 inhibitor • Diabetic nephropathy • CKD stage G5 • Shared decision making • Parachute induction
Introduction
The Sodium-Glucose Cotransporter 2 inhibitor (SGLT2i) is an antidiabetic drug that lowers blood glucose levels by increasing urinary glucose excretion. The clinical effectiveness of SGLT2i from the viewpoint of renoprotection in diabetic patients is widely recognized [1,2]. However, it is uncertain whether such effect can be expected even in the diabetic patients with CKD stage G5 severe kidney injury [3].
Here, we report our experience with respect to the prescription of the SGLT2i luseogliflozin in patients with CKD stage G5 Diabetic Nephropathy (DN).
Case Presentation
The subjects were 5 patients with severe kidney dysfunction probably due to DN who need to initiate Kidney Replacement Therapy (KRT) in the extremely near future, who received the first outpatient referral to the Department of Nephrology, Teikyo University Chiba Medical Center, between April 1, 2019 and March 31, 2020. Patients profile is summarized in Table 1. Although no patients underwent a kidney biopsy, they had (1) Diabetic retinopathy, (2) Apparent proteinuria, and (3) Elevation of urinary L-FABP (>55 μg/dL), and thus were diagnosed as DN [4].
| Factor | Group | Overall |
|---|---|---|
| n | 5 | |
| Sex | female | 3 (60.0) |
| male | 2 (40.0) | |
| Age (years) | 48.00 (45.00, 69.00) | |
| Serum creatinine (mg/dL) | 3.95 (3.07, 6.12) | |
| Estimated GFR (mL/min/1.73 m2) | 12.03 (6.18, 14.82) | |
| Period until KRT access creation (days) | 315.00 (60.00, 376.00) | |
| GFR; glomerular filtration ratio, KRT; kidney replacement therapy. | ||
Table 1. Patients profile.
To these patients, we administered 2.5 mg daily of SGLT2i luseogliflozin (Lusefi®, Taisho Pharmaceutical Co., Ltd., Tokyo, Japan) in addition to the already prescribed medicine to reduce the declining speed of kidney function and to win some time to select KRT modality under sufficient consideration through Shared Decision-Making (SDM) process. The reason we chose luseogliflozin as an administrative drug in these cases was that luseogliflozin is excreted not only by the kidney but also by the liver, and therefore the accumulation of luseogliflozin caused by kidney impairment in the body is mild [5]. As an indicator of kidney function, we adopted the estimated Glomerular Filtration Rate (GFR), which was calculated using the CKD-EPI formula in Japanese [6]:
mCKDEPI-eGFR (mL/min/1.73 m2=141 × min (Cr/κ, 1)α × max (Cr/κ, 1)-1.209 × 0.993 Age × 1.018 (if female) × 0.813 (Japanese coefficient), κ:0.7 in female and 0.9 in male, α was -0.329 in female and -0.411 in male.
We previously confirmed that the prognostic ability of eGFR calculated using the CKD-EPI formula is superior to that calculated using the estimation formula of the Japan society of nephrology [7].
Results
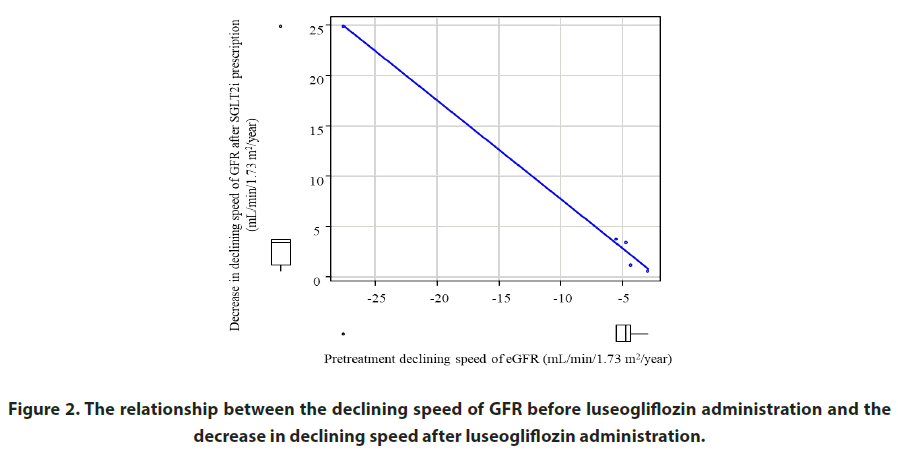
The declining speed of the GFR decreased in all cases. Figure 1 shows the change in the declining speed of kidney function after luseogliflozin administration. Figure 2 shows the relationship between the declining speed of GFR before luseogliflozin administration and the decrease in declining speed after luseogliflozin administration. The degree of decrease in declining speed of GFR was correlated with the declining speed prior to luseogliflozin administration.
Note: The declining speed of GFR decreased after luseogliflozin prescription in all cases.
Figure 2: The relationship between the declining speed of GFR before luseogliflozin administration and the decrease in declining speed after luseogliflozin administration.
Note: The degree of decrease after luseogliflozin administration was correlated with the declining speed of GFR before treatment.
In any case, an “initial decline” in kidney function just after luseogliflozin administration was not observed. Notably, all patients can select their favorable KRT modality through the SDM process (hemodialysis in 3 cases and peritoneal dialysis in 2 cases), and access to the KRT performance (arteriovenous fistula or embedded peritoneal dialysis catheter) was stabilized prior to the start of KRT.
Discussion
In our report of 5 patients with CKD stage G5 DN, the prescribing of the SGLT2i luseogliflozin reduced the decrease in kidney function and made it possible to win sufficient time to select KRT modality through SDM process and to avoid “parachute” KRT induction. Avoiding parachute or urgent KRT induction is important, since urgent KRT initiation, especially using a central venous catheter, relates to unfavorable prognosis [8]. In addition, the lack of sense regarding engagement in decision-making process results in poor satisfaction [9].
In all cases, no initial decline in GFR was found, which is commonly observed after starting SGLT2i treatment. This finding is consistent with a previous report by Inagaki et al. using Japanese long-term post-marketing surveillance data, which showed that no initial decline after the prescribing of SGLT2i (canagliflozin) was observed in patients with kidney dysfunction (estimated GFR <15 to <60 mL/min/1.73 m2) [3]. These could be because residual kidney function is too small to cause initial GFR decline through volume depletion in such cases.
Previously, we reported that the reduction in urinary protein excretion after SGLT2i among DN patients with impaired kidney function (CKD stage G4+G5) yields that with preserved kidney function (CKD stage G1+G2+G3) [10]. This finding suggests, theoretically, that the renoprotective potential of SGLT2i is expected even in patients with far advanced DN.
Several reports support the possibility of a tubule-centric concept, which claims that albuminuria among DN is caused not only by glomerulopathy, but also mainly by tubulopathy [11]. Previously, we compared the urine of DN patients with that of non-diabetic CKD patients and found that chemokine that represents tubular damage (CXCL5) increased only in DN patients in correlation with urinary albumin level [12]. From the viewpoint of tubule-centric concept above, our present finding suggests that SGLT2i administration could lessen tubule-intestinal damage in DN patients even in the far-advanced stage, as long as glomerular filtrate is present. SGLT2 belongs to the solute carrier family (SLC5A2), the action as a transporter simply depends on substrate concentration. The high concentration of glucose in blood (and thus in glomerular filtrate) could “force feed” proximal tubules over-absorption of glucose, just as the farmer force-feeds ducks and geese to make Foie gras. If that is the case, the administration of SGLT2i could rescue tubules and interstitial tissue from the Foie gras situation (=Foie gras hypothesis).
Conclusion
In summary, the present report suggests that the SGLT2 inhibitor luseogliflozin can reduce the speed of GFR decline even in patients with DN with severely impaired kidney function to the level of CKD stage G5. We believe that the use of the SGLT2 inhibitor in patients faced with urgent KRT induction can improve quality of life and prognosis after KRT initiation by winning sufficient time to select the KRT modality and avoiding parachute KRT induction.
References
- Tobe SW, Mavrakanas TA, Bajaj HS, et al. Impact of canagliflozin on Kidney and cardiovascular outcomes by type 2 diabetes duration: A pooled analysis of the CANVAS program and CREDENCE trials. Diabetes Care. 22, dc231450 (2024).
- EMPA-KIDNEY Collaborative Group. Effects of empagliflozin on progression of chronic kidney disease: a prespecified secondary analysis from the empa-kidney trial. Lancet Diabetes Endocrinol. 12, 39-50 (2024).
- Inagaki N, Nangaku M, Sakata Y et al. Treatment for type 2 diabetes mellitus in Japan: SAPPHIRE, a long-term, large-scale post-marketing surveillance. Adv Ther. 39, 674-691 (2022).
- Waki K, Iijima R, Nemoto Y, et al. Usefulness of L-FABP as a discriminative marker of diabetic nephropathy: A cross-sectional study. Teikyo Med J.
- Samukawa Y, Haneda M, Seino Y, et al. Pharmacokinetics and pharmacodynamics of luseogliflozin, a selective SGLT2 inhibitor, in Japanese patients with type 2 diabetes with mild to severe renal impairment. Clin Pharmacol Drug Dev. 7, 820-828 (2018).
- Horio M, Imai E, Yasuda Y, et al. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 56, 32-38 (2010).
- Terawaki H, Nakayama M, Asahi K, et al. Comparison of predictive value for first cardiovascular event between Japanese GFR equation and coefficient-modified CKD-EPI equation. Clin Exp Nephrol. 19, 387-394 (2015).
- Alizada U, Sauleau EA, Krummel T, et al. Effect of emergency start and central venous catheter on outcomes in incident hemodialysis patients: a prospective observational cohort. J Nephrol. 35, 977-988 (2022).
- Ladin K, Lin N, Hahn E, et al. Engagement in decision-making and patient satisfaction: a qualitative study of older patients’ perceptions of dialysis initiation and modality decisions. Nephrol Dial Transplant. 32, 1394-1401 (2017).
- Yoshimura K, Waki K, Iijima R, et al. SGLT2 inhibitor reduces urinary protein excretion even in the advanced diabetic kidney disease patients. Dis Disord. 2, 1-2 (2018).
- Comper WD, Hilliard LM, Nikolic-Paterson DJ, et al. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol. 295, F1589-1600 (2008).
- Higurashi M, Ohya Y, Joh K, et al. Increased urinary levels of CXCL5, CXCL8 and CXCL9 in patients with Type 2 diabetic nephropathy. J Diabetes Complications.; 23, 178-184 (2009).