Case Report - Journal of Neonatal Studies (2022) Volume 5, Issue 5
Late use of Surfactant in Neonatal Pneumonia
Muhammad Zaid Hamid Hussain1, Azka Feroz2, Kyle Damron3, Liaqat Hayat Khan3, Asif Z Khattak3*
1Department of Pediatrics, Aga Khan University Hospital, Karachi, Pakistan
2Dow University of Health Sciences
3Department of Pediatrics, Neonatology Division, Hunt Regional Hospital, Greenville, Texas
Received: 19-Sep-2022, Manuscript No. JNS-22-75092; Editor assigned: 20-Sep-2022; PreQC No. JNS-22- 75092 (PQ); Reviewed: 03-Oct-2022, QC No. JNS-22-75092; Revised: 07-Oct-2022, Manuscript No. JNS-22- 75092 (R); Published: 14-Oct-2022, DOI: 10.37532/jns.2022.5(5).79-83
Abstract
This case report presents a neonate born at 25+5 weeks of gestation suffering from Klebsiella pneumoniae Pneumonia during the third week of life resulting in an exacerbation of underlying respiratory distress syndrome. Surfactant was given on the 29th day of life on a compassionate basis yielding significant respiratory improvement.
Keywords
Klebsiella pneumoniae • Poractant alfa
Introduction
Pulmonary surfactant has proved to be a miracle in the field of neonatology. It has historically been used during the first few weeks of life to treat Respiratory Distress Syndrome (RDS). The widespread standard practice of administering surfactant in preterm neonates is evidence of how successful surfactant has been. Although alternate uses of surfactant have not been extensively explored, its role in treating neonatal pneumonia has previously been investigated. Deshpande and colleagues demonstrated a significant improvement in oxygenation after surfactant administration in late preterm and term neonates with pneumonia [1]. We present a unique case of neonatal pneumonia treated with late use of surfactant.
Case
A 30-year-old female, G2 P1, presented with preeclampsia and twin gestations to our maternal unit at 25+5 weeks of gestation. Due to worsening preeclampsia and twin gestation a decision was made to perform an urgent cesarean section. Subsequently twin A was delivered and was rushed to the warmer. The neonate was cyanotic with no audible cry and had poor tone. Despite suctioning and providing positive pressure ventilation to the patient, there was poor chest rise and reduced air bilaterally. Following the Neonatal Resuscitation Program protocol the neonate was intubated with 2.5 Endo Tracheal Tube (ETT). Oxygen saturations steadily increased, along with improvement in tone, color and heart rate. The APGARS at 1 and 5 minutes were 1 and 7, respectively. Once stabilized, the neonate was taken to the neonatal intensive care unit for further management.
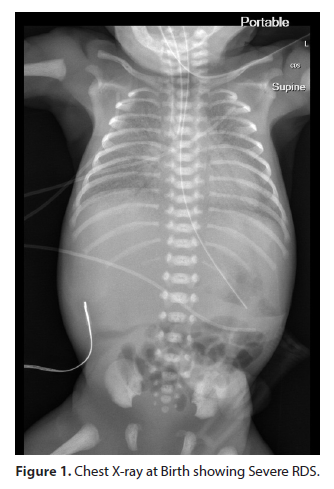
Birth weight was 0.890 kg (77.9 percentile), birth length was 33.0 cm (43.6 percentile) and birth head circumference was 24.5 cm (82.9 percentile). Chest X-ray revealed correct ETT placement, diffuse haziness and moderate reticular granular pattern) consistent with neonatal respiratory distress syndrome. The neonate was placed on High Frequency Oscillator Ventilation (HFOV). An initial dose of 2.5 ml/kg of Poractant alfa was given, with a second dose given at 12 hours of life. She was on standard antibiotics (ampicillin and gentamicin) for presumed sepsis from Day of Life (DOL) [1-5].
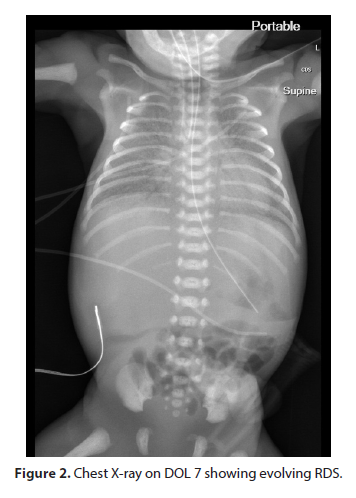
On DOL 7, there were frequent bradycardias and apneic events which required an increase in FiO2 requirement as well as resuscitation with Neopuff (Figures 1 & 2). Chest X-rays at this time revealed bilateral patchiness, resulting in the inability to rule out pneumonia although laboratory values, complete blood count and C-Reactive Protein (CRP), were in the normal ranges. A regimen of vancomycin and tobramycin were given for the next 48 hours (7-9 DOL). FiO2 requirements subsequently subsided with blood cultures showing no growth and antibiotics were discontinued. However, she remained on HFOV and presented with worsening lung disease. The neonate was given a two-week course of hydrocortisone.
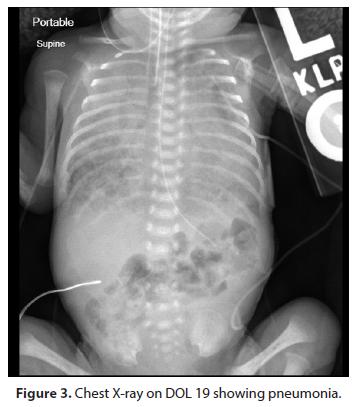
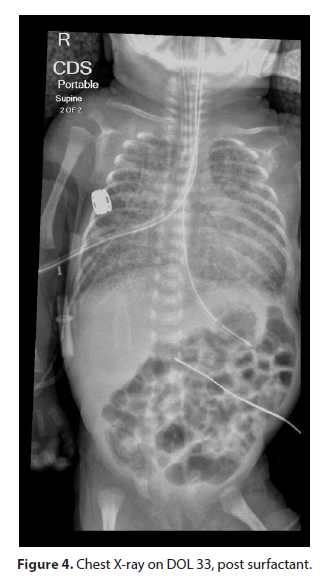
On DOL 19 Chest X-ray (Figure 3) showed a right upper lobe opacity leading to suspicion for pneumonia. The FiO2 requirements dramatically increased to 50-70%. She was reintubated with a size 3 ETT. A routine pneumonia work up with empiric treatment was ordered. She was started on vancomycin and tobramycin. Tracheal aspirate on DOL 20 showed heavy growth of gram-negative rods. In view of this finding, ceftazidime was added. On DOL 22 the tracheal aspirate was positive for Klebsiella pneumoniae which was susceptible to both tobramycin and ceftazidime. Vancomycin was subsequently discontinued. The CRP level on DOL 19 was 1.6 and it rose to 7.7 on DOL 24. On DOL 26, the FiO2 requirement dramatically increased to 100%. Tobramycin was discontinued and meropenem was initiated. For the following days, the FiO2 requirement was in the range of 98-100% maintaining O2 saturations in the 80s. On DOL 29, the patient failed a conventional ventilator trial, and she was placed back on the HFOV. At this point, following a family conference, a decision was made to give a course of Poractant allfa, on a compassionate basis, consisting of three doses, 12-24 hours apart. The FiO2 requirement fell to 65-67% by DOL 33. Chest X-ray (Figure 4) showed continued improvement, however chronic changes were still evident. In view of this improving lung condition, we decided to try to put the patient on SiPAP, however the patient needed to be reintubated after 20 minutes and placed back on HFOV. Antibiotics were discontinued on DOL 36 following resolution of the pneumonia on Chest X-ray.
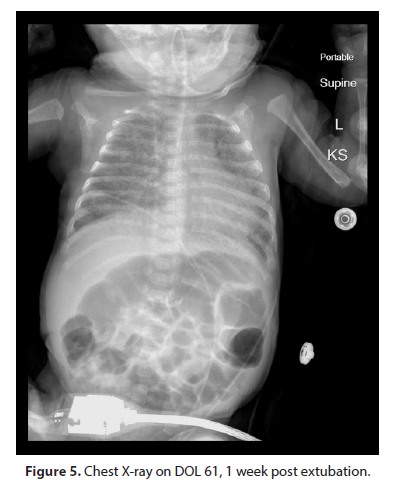
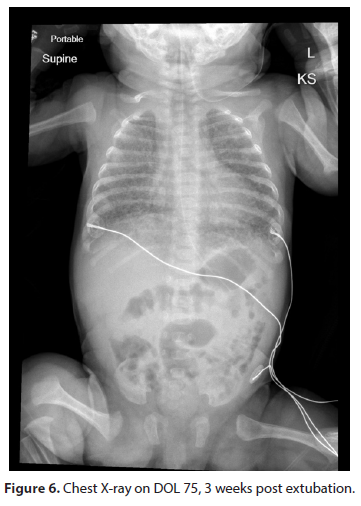
She was electively extubated again to SiPap on DOL 54 with FiO2 requirements then in the range of 40-50%. The patient continued to improve clinically. On DOL 62, she was successfully transitioned to nasal Continuous Positive Airway Pressure (nCPAP). She transitioned to high flow nasal cannula on DOL 68 and progressively weaned down to a flow rate of 1 L/min by DOL 128. The patient continued to grow and feed well. She was finally discharged on DOL 131 with a nasal cannula at 1 liters/ min. The patient had a discharge weight of 4.977 kg (50.0 percentile), length of 53.3 cm (4.5 percentile) and a head circumference of 38.5 cm (32.6 percentile). She was on full oral feeds and was breastfeeding. She was discharged home on a monitor and home oxygen. Serial neuroimaging done during NICU stay remained unremarkable. The patient’s Retinopathy of Prematurity (ROP) screening as per American Academy of Pediatrics guidelines showed Stage 1 ROP with mild plus disease, however serial exams showed regression and normal retinal vessel growth (Figures 5 & 6).
Discussion
This is a case of Klebsiella pneumoniae pneumonia in an extremely premature infant that developed in the third week after birth leading to secondary RDS, prompting the compassionate late use of surfactant that resulted in dramatic improvement in lung disease and survival. Various studies have established the unequivocal benefits of Surfactant Replacement Therapy (SRT) for neonates with RDS. Irrespective of the strategy or product used, the surfactant has been shown to decrease the need for ventilation support, risk of pulmonary air leak, mortality, and the combined outcome of death or Broncho Pulmonary Dysplasia (BPD) at 28 days, without increasing adverse neurodevelopmental outcomes [2].
Pulmonary surfactant has been studied from the 1980s with regards to the treatment and prevention of RDS in preterm infants. Surfactant is composed of ∼ 80-85% phospholipids, 5-10% neutral lipids and 8-10% protein, with 5-6% consisting of the four specific surfactant proteins. Pulmonary surfactant is essential for the function of gas exchange at the fluid-air interface of the alveolar surface. With its hydrophobic and hydrophilic properties, it decreases surface tension, thus keeping the alveoli open during the expiratory phase of the respiratory cycle. Exogenous surfactant administration in preterm infants quickly became the norm in treating RDS. Numerous subsequent trials in the past decades confirmed the effectiveness of surfactants in improving morbidity including the need for ventilation, short-term outcomes and mortality [3].
The surfactant proteins play an important role in respiratory immunity by facilitating the uptake of pathogens via phagocytic cells [4]. A preterm infant will have lower than normal surfactant levels. Additionally, the anatomical framework of the lung is immature. Both of these factors can lead to the preterm lung becoming a vulnerable site to respiratory pathogens resulting in pneumonia [5]. The relationship between pneumonia and surfactant has been much debated on Rudiger described how neonatal pneumonia further exacerbates this respiratory failure which is the probable reason why our patient presented with a very severe form of hypoxemic respiratory failure [6].
We explored the literature to see the use of surfactant in treating neonatal pneumonia. Studies have suggested that exogenous surfactant might be beneficial to infants with pneumonia [7]. An animal study conducted in 2004 showed how surfactant treatment resulted in significantly less bacterial growth with improved lung function [8]. Another study in 2017 concluded how there were marked improvements in oxygenation after surfactant therapy in late preterm and term neonates with pneumonia [1]. Keeping the basic pathophysiological processes in mind we decided to give exogenous surfactant to treat the respiratory decompensation in this infant on a compassionate basis. This was given on DOL 29 and showed significant improvements in the FiO2 requirement (98% to 60%) and the chest X-ray findings. However, there is no evidence from randomized controlled trials (RCTs) to support or refute the efficacy of surfactant in near-term and term infants with proven or suspected bacterial pneumonia [9]. RCTs are still required to answer this question.
Surfactant has generally been used within the first few days of life to treat RDS in preterm infants. The aim is to prevent a severe form of RDS from developing [10]. In our patient, we used surfactant in the traditional way after birth and decided to use it in the third week of life to treat secondary inactivation from pneumonia. In our literature review, we were unable to find any examples of surfactant used as late as we administered it. A retrospective study published in 2021 received surfactant at a median postnatal age of 8 days [11].
Conclusion
In our discussion we have highlighted how pneumonia can cause significant surfactant inactivation in an already, relatively surfactant deficient and developmentally immature lung environment. This case illustrates that the late use of surfactant in infants with severe respiratory distress and superimposed pneumonia, can prove to be a successful treatment. The onlabel usage of surfactant, specifically Poractant alfa, is within 48 hours of life. However, there are off-label indications with regards to the late use of surfactant which are prevention of bronchopulmonary dysplasia, refractory RDS or pneumonia [11]. Keeping these examples of the various benefits of the late use of surfactant in mind, further clinical studies should be conducted, specifically studies showing the role of surfactant in treating pneumonia.
Acknowledgement
None
Conflict of Interest
None
References
- Deshpande S, Suryawanshi P, Ahya K, et al. Surfactant Therapy for Early Onset Pneumonia in Late Preterm and Term Neonates Needing Mechanical Ventilation. J Clin Diagn Res. 11(8), SC09-SC12 (2017).
- Ng EH, Shah V. Guidelines for surfactant replacement therapy in neonates. Paediatr Child Health. 26(1), 35-49 (2021).
- Hentschel R, Bohlin K, van Kaam A, et al. Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr Res. 88(2), 176-183 (2020).
- Guagliardo R, Perez-Gil J, De Smedt S, et al. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J Control Release. 291, 116-126 (2018).
- Davis DJ, Barrington KJ. Recommendations for neonatal surfactant therapy. Paediatric Child Health. 10(2), 109-116 (2005).
- Rudiger M, Friedrich W, Rustow B, et al. Disturbed surface properties in preterm infants with pneumonia. Biol Neonate. 79(2), 73-78 (2001).
- Dargaville PA, Aiyappan A, De Paoli AG, et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology. 104(1), 8-14 (2013).
- Finer NN. Surfactant use for neonatal lung injury: beyond respiratory distress syndrome. Paediatric Respir Rev. 5, Suppl A:S289-297 (2004).
- Tan K, Lai NM, Sharma A. Surfactant for bacterial pneumonia in late preterm and term infants. Cochrane Database Syst Rev. (2), CD008155 (2012).
- Hartel C, Glaser K, Speer CP. The Miracles of Surfactant: Less Invasive Surfactant Administration, Nebulization, and Carrier of Topical Drugs. Neonatology. 118(2), 225-234 (2021).
- Lane MD, Kishnani S, Udemadu O, et al. Comparative efficacy and safety of late surfactant preparations: A retrospective study. J Perinatol. 41(11), 2639-2644 (2021).
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref