Case Report - Interventional Cardiology (2020) Volume 12, Issue 6
Hepatic cyst rupture: A hidden complication of chest compression
Yusuke Takeda, Hisayoshi Murai*, Soichiro Usui, Kenji Sakata, Masaaki Kawashiri, Masayuki Takamura
Department of Cardiovascular Medicine, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan
- Corresponding Author:
- Dr. Hisayoshi Murai
Department of Cardiovascular Medicine
Kanazawa University Graduate
School of Medical Sciences
Kanazawa, Japan
E-mail: sakurasoma1209@yahoo.co.jp
Received date: September 08, 2020 Accepted date: September 22, 2020 Published date: September 29, 2020
Abstract
Background: Chest compressions are absolutely essential to save the lives of those in cardiac arrest. However, chest compressions per se may induce certain complications. Here, we present a case of hepatic cyst rupture induced by chest compressions after ventricular fibrillation developed during percutaneous coronary intervention (PCI).
Case presentation: A 74-year-old male was admitted for PCI. Earlier computed tomography (CT) had incidentally revealed a large hepatic cyst. During PCI, ventricular fibrillation developed and the patient required cardiopulmonary resuscitation. Sinus rhythm recovery did not improve the hemodynamic stability. CT unexpectedly revealed an abdominal hematoma and extravasation of contrast medium from the ruptured hepatic cyst, causing hemorrhagic shock. We performed transcatheter arterial embolization, which stabilized the hemodynamics.
Conclusion: Chest compressions must be delivered carefully to patients at risk of hepatic cyst rupture. Careful consideration of adjacent organs around heart is required for prompt diagnosis and treatment to prevent serious shock.
Abstract
Background: Chest compressions are absolutely essential to save the lives of those in cardiac arrest. However, chest compressions per se may induce certain complications. Here, we present a case of hepatic cyst rupture induced by chest compressions after ventricular fibrillation developed during percutaneous coronary intervention (PCI).
Case presentation: A 74-year-old male was admitted for PCI. Earlier computed tomography (CT) had incidentally revealed a large hepatic cyst. During PCI, ventricular fibrillation developed and the patient required cardiopulmonary resuscitation. Sinus rhythm recovery did not improve the hemodynamic stability. CT unexpectedly revealed an abdominal hematoma and extravasation of contrast medium from the ruptured hepatic cyst, causing hemorrhagic shock. We performed transcatheter arterial embolization, which stabilized the hemodynamics.
Conclusion: Chest compressions must be delivered carefully to patients at risk of hepatic cyst rupture. Careful consideration of adjacent organs around heart is required for prompt diagnosis and treatment to prevent serious shock.
Keywords
Hepatic cyst rupture; Complication of chest compression; Hemorrhagic shock Cardiopulmonary resuscitation; Triple therapy Transcatheter arterial embolization
Abbreviations
PCI: Percutaneous Coronary Intervention; CT: Computed Tomography; CPR: Cardiopulmonary Resuscitation; LAD: Left Anterior Descending Artery
Background
Life-threatening cardiac arrhythmias are infrequently encountered in association with Percutaneous Coronary Intervention (PCI). During cardiopulmonary resuscitation (CPR), chest compressions are essential for survival. Although such compressions sometimes cause traumatic injuries, deeper compressions increase the success rate of spontaneous circulatory return [1,2]. Hepatic cysts are common benign lesions [3] that are usually asymptomatic, being discovered incidentally on ultrasonography and Computed Tomography (CT). Intraperitoneal cyst rupture is a rare complication. Most ruptures are caused by trauma, although spontaneous ruptures have been reported [4]. Here, we present a case of hepatic cyst rupture after chest compressions were delivered when ventricular fibrillation developed during PCI. We found no report on hepatic cyst rupture following chest compressions in the literature; our case is thus important.
Case Presentation
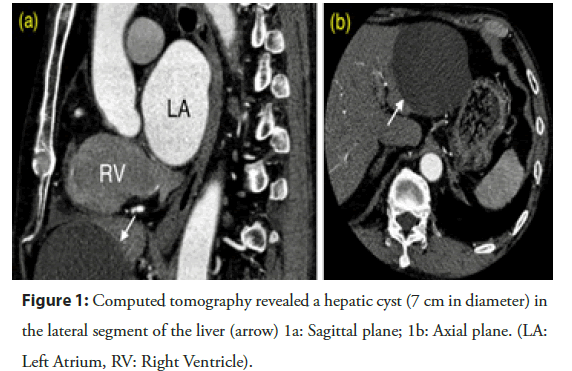
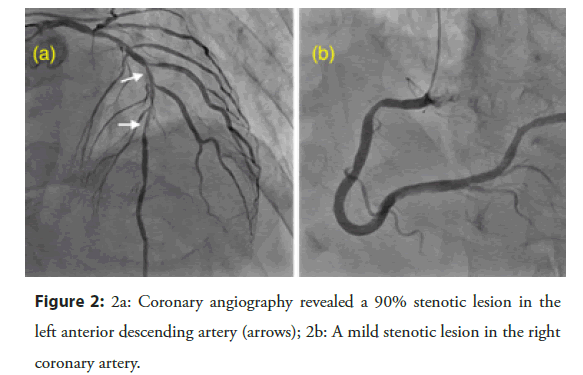
A 74-year-old male was admitted for PCI. His family history was unremarkable. Four months before admission he had undergone surgical thrombectomy to treat an acute embolic occlusion of the right brachial artery. At that time, an electrocardiogram and an echocardiogram revealed atrial fibrillation, mild enlargement of the left ventricle, reduced motion of the anterior septum, and an ejection fraction of 40%. Anticoagulation therapy with 60 mg/day edoxaban, a β-blocker, and an angiotensin-converting enzyme inhibitor were prescribed. Incidentally, a large hepatic cyst was detected in the lateral segment of the liver that lay close to the heart (across the diaphragm) by computed tomography (CT) (Figure 1). One month before admission, routine coronary angiography was performed to explore the etiology of left ventricular dysfunction. Stenosis was detected in the mid-left anterior descending artery (LAD) that was attributable to the left ventricular dysfunction (Figure 2). Thus, staged PCI was scheduled; we planned to address the stenosis. Dual antiplatelet therapy (aspirin and clopidogrel in addition to the direct oral anticoagulant; so-called ‘triple therapy’) was commenced.
Figure 2: 2a: Coronary angiography revealed a 90% stenotic lesion in the left anterior descending artery (arrows); 2b: A mild stenotic lesion in the right coronary artery.
| White blood cell(/μL) | 5520 | CRP(mg/dL) | 0.11 |
| Hemoglobin(g/dL) | 15.3 | CK(IU/L) | 168 |
| Platelet( × 10³/μL ) | 249 | ALP(IU/L) | 167 |
| PT(sec) | 17.2 | γ-GTP(IU/L) | 41 |
| APTT(sec) | 35.5 | AST(IU/L) | 20 |
| Fibrinogen(mg/dL) | 358 | ALT(IU/L) | 22 |
| Glucose (mg/dL) | 118 | LDH(IU/L) | 162 |
| Hemoglobin A1c(%) | 6.7 | T-Bil(mg/dL) | 0.5 |
| Sodium(mEq/L) | 140 | TP(g/dL) | 7 |
| Potassium (mEq/L) | 5 | T-Cho(mg/dL) | 156 |
| Chlorine(mEq/L) | 104 | LDL-Cho(mg/dL) | 96 |
| BUN(mg/dL) | 17 | HDL-Cho(mg/dL) | 39 |
| Creatinine(mg/dL) | 0.99 | Triglyceride(mg/dL) | 107 |
| BNP (pg/mL) | 191.9 |
*PT: Prothrombin time; APTT: Activated Partial Thromboplastin Time; BUN: Blood Urea Nitrogen; CRP: C-Reactive Protein; CK: Creatine Kinase; ALP: Alkaline Phosphatase; γ-GTP: γ-Glutamyl Transpeptidase; AST: Aspartate Transaminase; ALT: Alanine Transaminase; LDH: Lactate Dehydrogenase; T-Bil: Total Bilirubin; T-Cho: Total Cholesterol; LDL-Cho: LDL- Cholesterol; HDL-Cho: HDL- Cholesterol; BNP: Brain Natriuretic Peptide
Table 1: Laboratory data on admission.
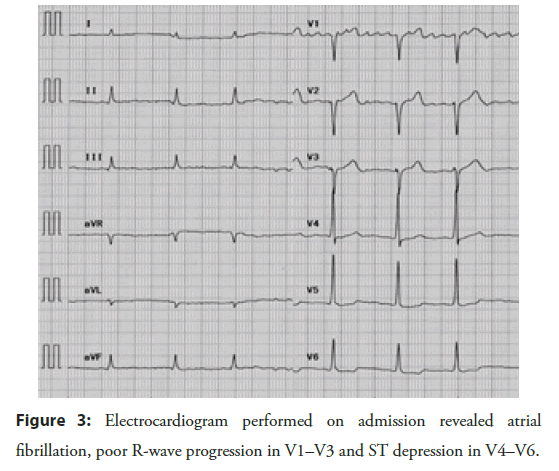
On admission, the patient’s blood pressure was 126/76 mmHg, his heart rate was 62 beats/min, and his SpO2 was 98% on room air. The conjunctiva showed no sign of anemia or jaundice. The first and second heart sounds were normal. A pan-systolic murmur was evident at the cardiac apex (Levine 2/6). No lung rales were audible. The abdomen was flat and soft; the liver was not palpated. We noted no edema. As shown in the Table 1, laboratory tests revealed mild renal dysfunction and an elevated level of brain natriuretic peptide. There was no sign of an inflammatory response, anemia, or liver dysfunction. A chest X-ray revealed a cardiothoracic ratio of 56.9% and no evidence of pleural effusion. The electrocardiogram revealed atrial fibrillation, poor R-wave progression in V1–V3, and ST depression in V4–V6 (Figure 3).
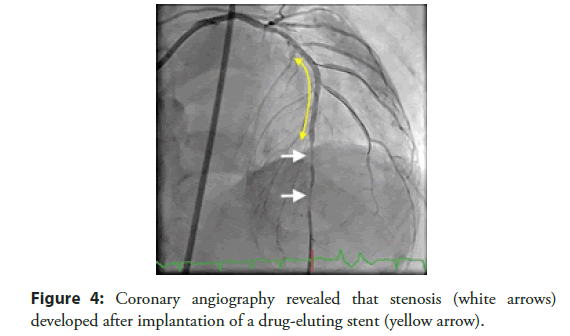
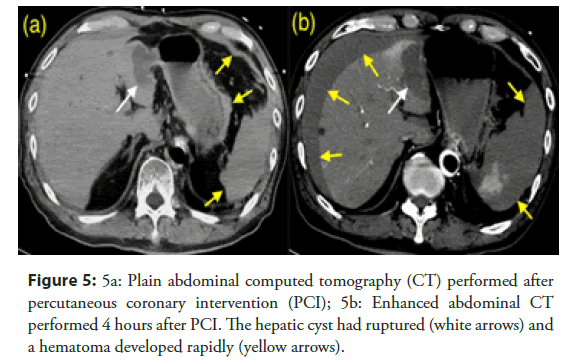
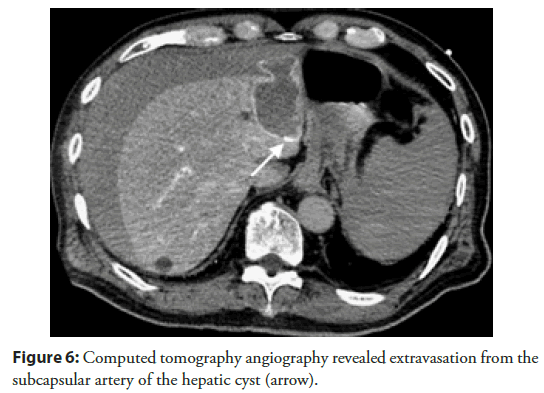
On hospital day 3, we performed PCI. A 7-Fr sheath was inserted into the right femoral artery and 8,000 U of heparin sodium were intravenously injected. After predilation with a 2.0 mm balloon at nominal pressure, a 2.5 mm zotarolimus-eluting stent was implanted at nominal pressure. Immediately after implantation, the patient complained of chest pain. Although isosorbide dinitrate was injected into the left coronary artery, coronary angiography revealed severe stenosis at the distal end of the stent, and coronary vasospasm (Figure 4). Suddenly, the electrocardiogram reported ventricular fibrillation. First, we performed CPR while we prepared for direct current cardioversion; 150-J direct-current cardioversion converted the ventricular fibrillation into a normal sinus rhythm. The angiographic stenosis in the LAD resolved, but the patient’s blood pressure indicated shock. An intra-aortic balloon pump was immediately inserted, but the blood pressure did not recover. We suspected occult bleeding; abdominal CT revealed a very small intra-abdominal hematoma and hepatic cyst rupture (Figure 5a). We felt that this explained the fall in blood pressure. We first employed conservative treatment; we discontinued the heparin infusion. However, the patient became gradually hypotensive and required large volumes of intravenous fluids and catecholamine. Enhanced CT of the abdomen revealed that the hematoma had grown; contrast medium was extravasated from the ruptured hepatic cyst (Figure 5b). We performed emergency angiography and transcatheter arterial embolization. CT angiography demonstrated extravasation from small arterial branches suppling the hepatic cyst (Figure 6). We embolized these arteries using gelatin sponges; the hemodynamics became stable. The hematoma gradually reduced and was barely visible after 1 week. Three weeks after the event, the patient was discharged in good health.
Figure 4: Coronary angiography revealed that stenosis (white arrows) developed after implantation of a drug-eluting stent (yellow arrow).
Figure 5: Plain abdominal computed tomography (CT) performed after percutaneous coronary intervention (PCI); 5b: Enhanced abdominal CT performed 4 hours after PCI. The hepatic cyst had ruptured (white arrows) and a hematoma developed rapidly (yellow arrows).
Figure 6: Computed tomography angiography revealed extravasation from the subcapsular artery of the hepatic cyst (arrow).
Discussion
Simple hepatic cysts are common benign lesions; the population frequency is about 2.5% [3]. The cysts are usually asymptomatic, being incidentally discovered during abdominal ultrasonography or CT. Rarely, cysts cause abdominal symptoms via the compression of adjacent organs. Asymptomatic hepatic cysts do not require treatment. If a cyst is symptomatic, percutaneous aspiration, sclerosis with alcohol or minocycline, or laparoscopic deroofing, is performed [5-7].
Hepatic cyst rupture is a rare complication, triggering sudden abdominal pain. Rupture may be caused by trauma or infection, but it is sometimes spontaneous [4]. No definitive treatment is available. Earlier case reports employed conservative strategies, including abdominal drainage, surgery, and transcatheter arterial embolization [8,9]. In our present case, the abdominal hematoma grew rapidly and the hemodynamic status became unstable; intervention was thus required. As CT revealed extravasation of the contrast medium, and as the effects of triple antiplatelet therapy persisted, we chose transcatheter arterial embolization, not surgical intervention. In our case, triple heparin antiplatelet therapy may have contributed to the hemorrhagic shock; hepatic cyst rupture rarely induces such shock. On the other hand, there are some cases that hepatic cysts induced cardiac compression and caused hemodynamic instability [10,11]. From these cases and our case, the heart and the liver are in proximity and an abnormality in one may affect the other.
Patients in cardiopulmonary arrest require CPR. The European Resuscitation Council and the American Heart Association have published relevant guidelines. Pressure should be applied to the lower half of the breastbone; it is important to avoid compressing the upper abdomen or the bottom of the bony sternum [1,2]. However, CPR-related complications have been described [12,13].
Abdominal complications develop in about 30% of those who undergo CPR [14]. In our case, it is likely that the hepatic cyst was injured via chest compression slightly caudal to the sternal bone. As a 7-cm-diameter hepatic cyst was noted prior to PCI, we should have performed chest compression in a more superior position. However, the placement of the X-ray tube and plate detector during PCI disturbed the number of sites available for chest compression.
Conclusion
We experienced a case of hepatic cyst rupture caused by chest compressions during PCI. Such compressions increase the risk of hepatic cyst rupture. Careful consideration of adjacent organs around heart is required for prompt diagnosis and treatment to prevent serious cardiogenic shock and secondary hemorrhage.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to express their appreciation to all those who provided their assistance in the completion of this case report.
References
- Perkins GD, Handley AJ, Koster RW, et al. European resuscitation council guidelines for resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation. 95: 81-99 (2015).
- Travers AH, Perkins GD, Berg RA, et al. Part 3: Adult basic life support and automated external defibrillation: 2015 International Consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 132: S51-83 (2015).
- Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol. 62(736): 335-337 (1989).
- Poggi G, Gatti C, Delmonte A, et al. Spontaneous rupture of non-parasitic hepatic cyst. Int J Clin Pract. 60(1): 99-103 (2006).
- Koperna T, Vogl S, Satzinger U, et al. Nonparasitic cysts of the liver: results and options of surgical treatment. World J Surg. 21(8): 850-854 (1997).
- Yoshida H, Onda M, Tajiri T, et al. Long-term results of multiple minocycline hydrochloride injections for the treatment of symptomatic solitary hepatic cyst. J Gastroenterol Hepatol. 18(5): 595-598 (2003).
- Yoshida H, Egami K, Onda M, et al. Treatment of symptomatic hepatic cyst by injection of minocycline hydrochloride. J Hepatobiliary Pancreat Surg. 3: 491-4 (1996).
- Mori T, Abe N, Sugiyama M, et al. Laparoscopic hepatobiliary and pancreatic surgery: an overview. J Hepatobiliary Pancreat Surg. 9(6): 710-722 (2002).
- Fong ZV, Wolf AM, Doria C, et al. Hemorrhagic hepatic cyst: report of a case and review of the literature with emphasis on clinical approach and management. J Gastrointest Surg. 16(9): 1782-1789 (2012).
- Abdullah A, Serdar Ö, Mehmet S, et al. Cardiac compression of a hepatic cyst in polycystic liver disease: A rare cause of hemodynamic instability. Turk J Emerg Med. 20(2): 93-96 (2020).
- Tuyen LV, Truong VT, Bui QVP, et al. Hepatic cysts induced extra-cardiac compression of right atrium: an uncommon cardiac complication. J Am Coll Cardiol. 75(11): S1 (2020).
- Kim MJ, Park YS, Kim SW, et al. Chest injury following cardiopulmonary resuscitation: a prospective computed tomography evaluation. Resuscitation. 84(3): 361-364 (2013).
- Hellevuo H, Sainio M, Nevalainen R, et al. Deeper chest compression-More complications for cardiac arrest patients? Resuscitation. 84(6): 760-765 (2013).
- Krischer JP, Fine EG, Davis JH, et al. Complications of cardiac resuscitation. Chest. 92(2): 287-291 (1987).