Case Report - Clinical Practice (2019) Volume 16, Issue 2
Erectile and ejaculatory dysfunction improvement related to spinal cord injury recovery after combination approach of rehabilitation and mesenchymal stem cell implantation
- Corresponding Author:
- Ahmad Jabir
Rahyussalim
Department of Orthopaedic and Traumatology
Faculty of Medicine Universitas Indonesia - Cipto Mangunkusumo General Hospital, Jakarta, 10430, Indonesia
E-mail: rahyussalim71@ui.ac.id
Abstract
Spinal Cord Injury (SCI) is a devastating condition that often leads to permanent functional and neurological deficits in injured individuals. One of the most overlooked consequences of spinal cord injury is sexual functions, such as erectile and ejaculatory dysfunction. Mesenchymal stem cells (MSCs) have been reported as adjunctive treatment to SCI cases. Some animal studies reported both functional and conductivity improvement after MSCs application. Clinical studies on human has also been conducted in small basis, but so far showed equivocal results.
Keywords
Erectile dysfunction, spinal cord injury, mesenchymal stem cells
Case Illustration
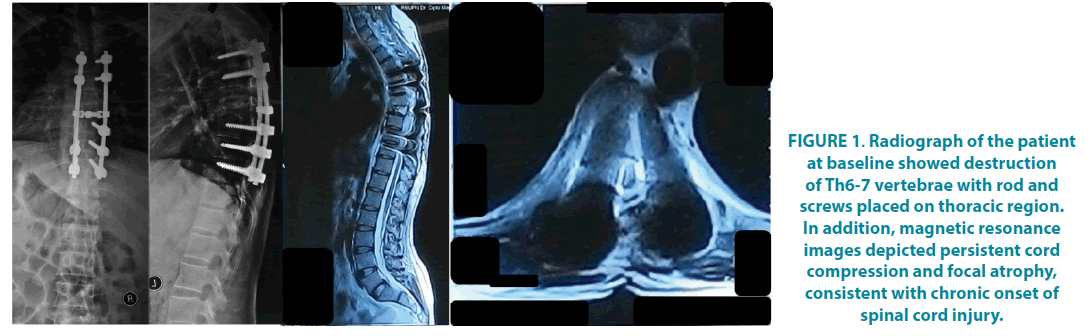
A thirty-seven-year-old male patient was riding a motorcycle at moderate speed (30-40 km/hour) when he was hit by a car from the right side six years before visiting us in our spine clinic. He had no sensory or motor function at the level of Th7 and below. He was also examined for Somatosensory Evoked Potential (SSEP), which found a total functional lesion of dorsal horn of spinal cord at both sides of C7-Th12 vertebrae (FIGURE 1). Motor Evoked Potential (MEP) examination revealed that central interneuron activity in right side was lower than left side. He also complained of erectile dysfunction and retrograde ejaculation, which was measured by Erection Hardness Score (EHS) and International Index of Erectile Function (IIEF). At the baseline, he had EHS of 1 (penis is larger and not hard) and IIEF of 8 (moderate erectile dysfunction). His poor erectile function was also showed by rigiscan that stated an organic erectile dysfunction due to neurological problem. In addition, he had no ejaculation.
Figure 1. Radiograph of the patient at baseline showed destruction of Th6-7 vertebrae with rod and screws placed on thoracic region. In addition, magnetic resonance images depicted persistent cord compression and focal atrophy, consistent with chronic onset of spinal cord injury.
Here, we had planned a cycle of stem cell application, consisting of three consecutive injections with interval of 2 weeks. For first injection, we supplied 10 × 106 cells via intravenous route, 16 × 106 cells via intrathecal route at inter laminar space of vertebrae lumbar 3 and 4, and 16 × 106 cells via intra-lesion route at level vertebrae thoracic 7. The second and third injection was given16 × 106 cells by intralesion fashion. The stem cells were cultured from his own bone marrow.
At one-month follow-up, his erection function improved by EHS score of 2 (Penis is hard, but not hard enough for penetration) and 13 (Mild to moderate erectile dysfunction) respectively (TABLE 1). Clinically there were also sensory improvement, Normal sensory function was found at the level of Th7-8 and hypoesthesia was found at the level of Th9 and below. At this time, minimally active movement of the hip flexor muscles and palpable contraction of the knee extensor muscles were also noted. At three-month follow-up, he experienced a successful ejaculation, albeit an inadequate one. He had no orgasm. Moreover, his erectile function improved further, as showed by EHS score of 3 (Penis is hard enough for penetration, but not completely hard) and IIEF score of 21 (Mild erectile dysfunction).
| Evaluation | Baseline | 1 month | 3 months |
|---|---|---|---|
| Sensory function (SSEP) | Total functional lesion of bilateral dorsal horn of C7-Th12 | N/A | Total functional lesion of bilateral dorsal horn of C7-Th12 |
| Motor function (MEP) | Central interneuron activity in right side is lower than left side | N/A | Central interneuron activity in right and left side is moderate |
Erectile function
|
1 8 |
2 13 |
3 21 |
| Ejaculation function | No ejaculation | No ejaculation | Normal ejaculation |
Table 1: Nerve and erectile function follow up.
Discussion
Long tracts of the central nervous system between the cortex and sacral spinal cord need to be intact for the spinal cord and preservation of his type of responsiveness in men with low spinal cord physiological sexual function. The cerebral centers which have important role in erection mechanism have not yet been well defined. Spinal cord damage can inhibit the transmission of impulses down the spinal cord and through the peripheral and autonomic nervous system to the genitalia.
Erection is a complex neuro-vascular event. In peripheral innervation, the pelvic plexus has a great role as a junction for efferent nerves to the structures involved in erection and ejaculation. There are three sets of neurons at the spinal cord which innervate the sexual organs involved in erection and ejaculation, namely thoracolumbar sympathetic, sacral parasympathetic, and somatic. The brain has an excitatory or inhibitory effect on the process of erection and ejaculation by the presence of cerebral descending pathway.
There are several structures responsible for three types of erection: psychogenic, reflexogenic, and nocturnal. Psychogenic erection can be achieved by audiovisual stimuli or fantasy. The brain sends impulses to modulate the spinal erection centers (T11-L2 and S2-S4) to activate the erectile process. While, reflexogenic erection is achieved by tactile stimuli to the genital organs. These impulses modulate the spinal erection centers and then follow the ascending tract, resulting in sensory perception. Some other impulses activate the autonomic nuclei to activate the cavernous nerves to the penis to induce erection. Reflexogenic erection is preserved in patients with upper spinal cord injury. Nocturnal erection happens mostly during Rapid-Eye-Movement (REM) sleep.
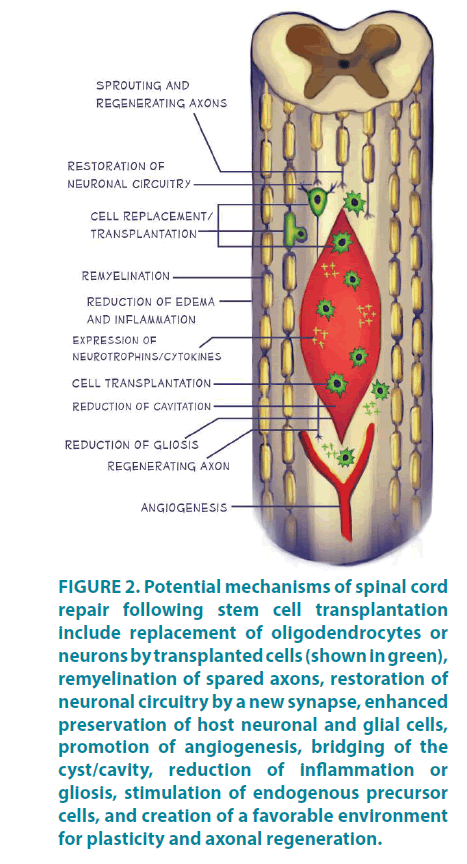
In the present case, spinal cord injection led to improvement on both erectile and ejaculatory function of the patient, as soon as 1-month follow-up. Several mechanisms for recovery have been proposed, including replacement of oligodendrocytes or neurons, remyelination of spared axons, restoration of neuronal circuitry, enhanced preservation of host neuronal and glial cells, increased expression of neurotrophins/ cytokines by transplanted or host cells, promotion of angiogenesis, bridging of cysts or cavities, reduced inflammation or gliosis, stimulation of endogenous precursor cells, and creation of a favorable environment for plasticity and axonal regeneration (FIGURE 2). In most studies, the exact mechanisms of improvement were not completely defined.
Figure 2: Potential mechanisms of spinal cord repair following stem cell transplantation include replacement of oligodendrocytes or neurons by transplanted cells (shown in green), remyelination of spared axons, restoration of neuronal circuitry by a new synapse, enhanced preservation of host neuronal and glial cells, promotion of angiogenesis, bridging of the cyst/cavity, reduction of inflammation or gliosis, stimulation of endogenous precursor cells, and creation of a favorable environment for plasticity and axonal regeneration.
It has been known that sexual function in different stages after SCI and the types of erections depend mainly on the completeness of the injury and the level of neurological damage. The patient in this case report had a complete SCI at the level of Th7 vertebrae. He had poor remaining erectile and ejaculatory function, as depicted by initial EHS and IIEF score as well as rigiscan examination. For complete SCI, both erectile and ejaculatory problems was mainly associated with neurologic injury that causes autonomic control dysfunction as well as inadequate brain stimulus toward effector side [1,2].
The residual erectile capacity of spinal cord injured patients is a function of the remaining neural connections within the genital system. The anatomical connections within the genital system include two nervous pathways, one originating from the sacral segments S2, S3, S4 of the spinal cord and involving the pudendal and pelvic nerves, the other originating from the thoracic-lumbar segments T11, T12, L1, L2 and involving the hypogastric, sympathic, and pelvic nerves.
The first of these two pathways, namely the sacral pathway, has been studied extensively at the anatomical and physiological level, as well as at the animal and human level. At the spinal cord injury level it has been demonstrated that the thoracic-lumbar (TL) pathway could convey penile responses forom stimulation of higher CNS structures in the absence of reflex activity from the sacral pathway. At the animal level, cauda equine lesions of S1 completely interrupted reflex activity from the sacral pathway, including bladder function and penile responses to genital stimulation. Nevertheless, 85% of the animals maintained penile responses to stimulation of a hypothalamic area, a support for the hypothesis of a role for the TL pathway in mediating erection. At the human level, 80% of the subjects whose lesions interrupted the sacral pathway maintained erectile responses to psychogenic stimulation. These findings, along with the previous ones, further emphasize the complementary role of the TL pathway in paraplegic subjects who have lost function from the sacral pathway. The overall results show that very few spinal cord injured subjects suffer from complete impotence. The vast majority maintain a residual erectile capacity upon which a sexual rehabilitation programme could be build.
Patients with sacral SCI retain psychogenic erectile ability even though reflexogenic erection is abolished. These cerebrally elicited erections are found more frequently in patients with lower motor neuron lesions below T12. However, no psychogenic erection occurs in patients with lesions above T9, as seen in our patient, because the efferent sympathetic outflow is located at the levels T11 and T12 [3]. Also reported, in these patients with psychogenic erections, lengthening and swelling of the penis are observed but rigidity is insufficient [4].
He experienced immediate improvement on both erectile and ejaculatory manifestations, slightly before improvement on sensory and motor function. There were only few cases of chronic SCI that found its way to clinical improvement [5] and furthermore, to our knowledge, there was still no published article describing the recovery of sexual function of SCI patients. Furthermore, it was widely known that both parasympathetic and sympathetic component in the spinal cord are located centrally. The patient had complete transection of his spinal cord with persistent cord compression and focal atrophy. Even though it was not proved by imaging modality, there was an immediate recovery of sexual functions, which is known to be driven mainly by autonomic functions. From this finding, we hypothesized that the prompt recovery of erectile and ejaculatory functions in our patients was due to central healing of spinal cord that involves sacral parasympathetic pathway.
The sympathetic and parasympathetic nervous systems act in synergy to activate physiological events occurring during ejaculation. Both sympathetic and parasympathetic tones are under the influence of sensory genital and/or cerebral erotic stimuli integrated and processed at the spinal cord level.
Sympathetic, parasympathetic, and somatic nerves originating in lumbosacral spinal nuclei command the peripheral anatomical structures responsible for ejaculation. Sensory afferents originating in genital areas are integrated at the spinal and brain levels. Activity of spinal preganglionic and motor neurons is under the influence of peripheral and supraspinal inputs.
Recently, there was a study about Spinal Ejaculation Generator (SEG) which has been identified to have a pivotal role in the control of ejaculation. Galaninergic neurons were mostly located between L2 and L5 segments in medial lamina VII, with a maximal density within L4. In addition, galaninergic neuron density was found higher in L3 and L4 segments in men as compared to women supporting sexual dimorphism. In the patients’ cohort, injury of L3-L5 segments was the sole independent predictor for failure of Penile Vibratory Stimulation (PVS) to induce ejaculation.
Conclusion
This report provided a novel insight to regenerative model of chronic SCI patients as well as further addition of successful MSCs treatment for chronic SCI cases.
References
- Volarevic V, Erceg S, Bhattacharya SS, Stojkovic P, Horner P, Stojkovic M. Stem cell-based therapy for spinal cord injury. Cell Transplant. 22(8), 1309-1323 (2013).
- Falavigna A, Finger G, de Souza OE, Pasqualotto FF. Spinal cord injury and male infertility: a review. Columna. 11(4), 322-325 (2012).
- Bors E, Camarr A. Neurological distubances in sexual function with special reference to 529 patients with spinal cord injury. Urol. Surv. 10, 191 (1960).
- Lane MA, Lepore AC, Fischer I. Improving the therapeutic efficacy of neural progenitor cell transplantation following spinal cord injury. Expert Rev. Neurother. 17(5), 433-440 (2017).
- Rahyussalim AJ, Djaja YP, Saleh I, Safri AY, Kurniawati T. Preservation and Tissue Handling Technique on Iatrogenic Dural Tear with Herniated Nerve Root at Cauda Equina Level. Case Rep. Orthop. (2016)
- Volarevic V, Erceg S, Bhattacharya SS, Stojkovic P, Horner P, Stojkovic M. Stem cell-based therapy for spinal cord injury. Cell Transplant. 22(8), 1309-1323 (2013).
- Falavigna A, Finger G, de Souza OE, Pasqualotto FF. Spinal cord injury and male infertility: a review. Columna. 11(4), 322-325 (2012).
- Bors E, Camarr A. Neurological distubances in sexual function with special reference to 529 patients with spinal cord injury. Urol. Surv. 10, 191 (1960).
- Lane MA, Lepore AC, Fischer I. Improving the therapeutic efficacy of neural progenitor cell transplantation following spinal cord injury. Expert Rev. Neurother. 17(5), 433-440 (2017).
- Rahyussalim AJ, Djaja YP, Saleh I, Safri AY, Kurniawati T. Preservation and Tissue Handling Technique on Iatrogenic Dural Tear with Herniated Nerve Root at Cauda Equina Level. Case Rep. Orthop. (2016)