Device Evaluations - Interventional Cardiology (2012) Volume 4, Issue 3
Endurant stent graft: a newgeneration device for complex endovascular aortic aneurysm repair
- Corresponding Author:
- Nicola Troisi
Department of Vascular Surgery, St Franziskus Hospital, Münster, Germany
Tel: +49 0251935 3933
Fax: +49 0251935 4092
E-mail: nicola.troisi@alice.it
Abstract
Keywords
abdominal aortic aneurysm, endograft, endovascular aneurysm repair, endurant stent graft
Since its introduction in 1991 [1], endovascular aortic aneurysm repair (EVAR) has played an increasing role in the management of abdominal aortic aneurysm (AAA). Accordingly, well-known randomized, controlled trials [2–5] have been designed to describe the early and long-term results of early aortic endografts.
These trials demonstrated that EVAR decreases perioperative morbidity and mortality, blood loss and the length of the stay at the intensive care unit and in hospital. In addition, endovascular techniques make it possible to treat patients whose comorbidities make conventional open repair difficult or risky.
For these reasons, many physicians feel that high-risk patients with AAA should be preferentially treated by EVAR. However, the first- and second-generation devices showed well-recognized limitations and their use has been limited to patients with specific anatomic selection criteria: proximal aortic neck longer than 15 mm; proximal aortic infrarenal angulation less than 60°; adequate diameters of the iliofemoral axis (≥7 mm); absence of circumferential iliac calcifications; and absence of severe iliac tortuosity.
In particular, severe infrarenal aortic neck angulation is strongly associated with subsequent proximal Type I endoleak [6] and dilatation of the proximal aortic neck represents a clear risk factor for endograft migration [7]. In patients with angulated or dilated proximal aortic necks, proximal graft sealing still remains a challenge and EVAR using a flexible stent graft seems to be feasible in patients with significantly angulated necks [8].
Furthermore, access-related difficulties, mainly due to tortuous, small and calcified iliac vessels, also continue to represent a major problem for EVAR [9]. Several strategies have been reported in the literature to overcome access problems and limit arterial access complications [10,11]; however, the main factor to allow the safe passage of the graft through the iliac vessels is generally considered to be the diameter and traceability of the delivery system.
Endurant® stent graft (Medtronic Cardiovascular, CA, USA) was recently developed with the aim of overcoming some of these limitations and to expand the indications for EVAR [12]. The graft appears to be efficacious and safe for the endovascular management of AAAs in patients with hostile anatomy of the proximal aortic neck and iliac arteries [13–18].
Endurant stent graft
The Endurant stent graft is comprised of polyester graft material externally supported by an electropolished nitinol stent structure. The Endurant stent graft offers a variety of components and sizes to treat several different anatomies. Endurant is comprised of abdominal tubes, aortic extension stent grafts, bifurcated stent grafts, contralateral limbs and iliac extensions.
The graft is a woven structure of multi-filament polyester yarn, which can be woven into shape to avoid the use of longitudinal seams. A double seam is present on the flared ipsilateral limbs of the bifurcated devices. The bifurcated devices have universal docking gates, which works with all sizes of the contralateral limb for a simplified sizing matrix. The aortic sections of the bifurcated stent graft components should be oversized by approximately 10–20% in relation to the actual measured vessel diameter. The contralateral limb and iliac extension component should be oversized by approximately 10–25% in relation to the vessel diameter.
The suprarenal stent is laser cut with anchoring pins near the proximal end as a part of the nitinol structure. The remaining part of the stents are formed from nitinol wire. The wire-formed M-shaped body stents provide conformability and flexibility during deployment. The nitinol is attached to the graft material by sutures. To ensure the contralateral gate is accessible in angulated situations, the limbs are sewn together between the first stents of the stub and iliac.
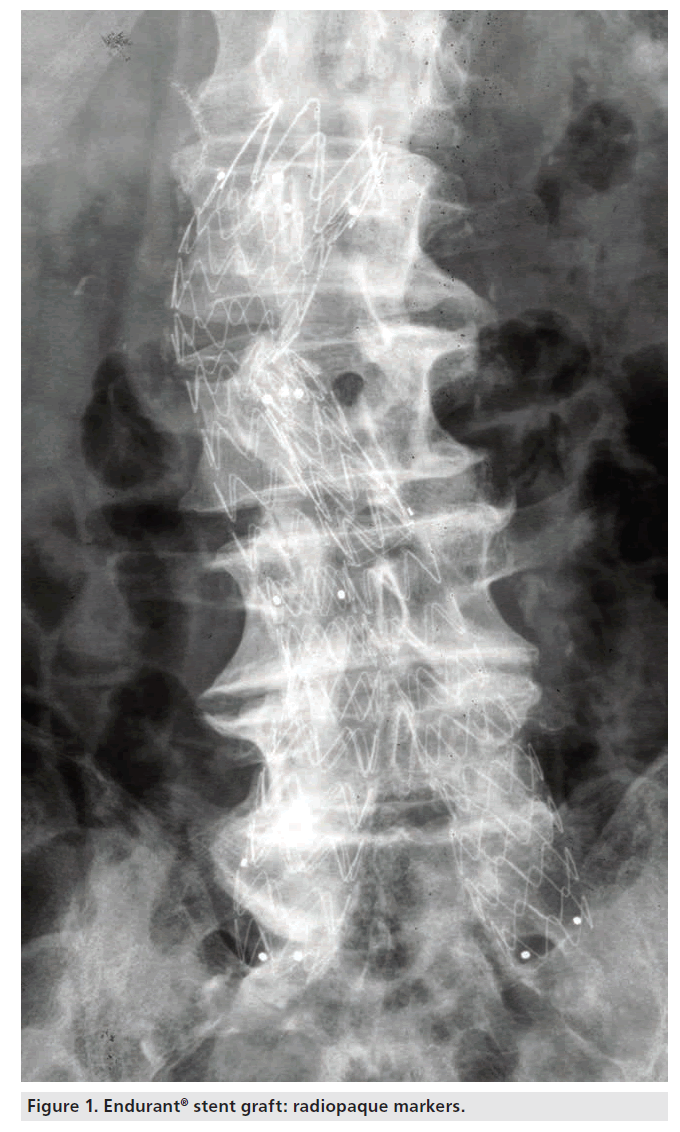
Radiopaque markers are sewn onto the stent graft to aid visualization and to facilitate accurate placement of the device. The radiopaque markers are placed at the proximal and distal ends of all stent graft segments, as well as at the bifurcation of the bifurcated stent grafts, indicating the edges and locations of the stent grafts (Figure 1). The nitinol stents may also be visualized under fluoroscopy.
The Endurant stent graft is loaded inside the Endurant delivery system, which facilitates the placement of the stent graft via the arterial vasculature (e.g., femoral arteries), ranging from 18 to 20 F for the main body and from 14 to 16 F for the iliac extensions. Using fluoroscopic guidance, the Endurant delivery system is properly positioned within the patient’s vasculature and the stent graft is deployed from the Endurant delivery system.
The Endurant delivery system consists of a single use, disposable catheter with an integrated handle to provide the user with accurate and controlled deployment. The catheter assembly is flexible and compatible with a 0.035 inch guidewire.
The Endurant delivery system is constructed of four concentric single lumen shafts (an outer polymer graft cover sheath with a hydrophillic coated graft cover, a polymer middle member shaft, a stainless steel spindle-tube shaft and a nitinol inner member guidewire lumen). A stent stop is attached to the distal end of the middle member shaft to ensure the Endurant stent graft position during deployment. The Endurant delivery system features a tipcapture mechanism to ensure the accurate control–release deployment of suprarenal stents.
A polymeric, atraumatic tapered tip is attached at the distal end of the inner member to facilitate tracking through tortuous and calcified vessels. Attached to the polymeric tip is a metallic sleeve that constrains the suprarenal stent onto the spindle. The polymeric tapered tip, stent stop and marker on the distal end of the graft cover are radio-opaque and aid in fluoroscopic visualization. Hemostasis is maintained by various components within the delivery system, preventing blood loss during the procedure. Retraction of the graft cover while the suprarenal stent is held in place by the spindle allows for accurate deployment of the covered stent graft portion.
Postdeployment, the physician needs to reseat the tip of the delivery system before returning the tip to the graft cover and removing the delivery system from the body.
Clinical results
The design of stents and the delivery system allows the treatment of more complex proximal aortic necks, overcoming some strict limitations of prior devices. The manufacturer’s instructions for use (IFU) recommend a proximal aortic neck ≥15 mm in length with ≤75° infrarenal angulation or a proximal aortic neck ≥10 mm with ≤60° infrarenal angulation.
In our first published multicenter experience [13], the Endurant stent graft allowed us to treat AAAs with very short necks (<10 mm), guaranteeing good outcomes in the early term. In a second published report [14], a third of patients had a neck <10 mm and approximately 40% of patients had a severely angulated neck (>60°); in this series, we achieved excellent outcomes both in the early (1.3% Type I endoleak rate) and mid-term periods (96.9% of freedom from Types I/III endoleak at 2 years). Furthermore, our analysis of factors affecting the long-term incidence of Types I/III endoleak found that angulation was not a risk factor. Rather, an aortic neck ≤10 mm, neck thrombus and use of a chimney technique affected the rate of Types I/III endoleak during follow-up. In this series, the key factor for the durability of the procedure was not the angulation but the shortness of the proximal aortic neck.
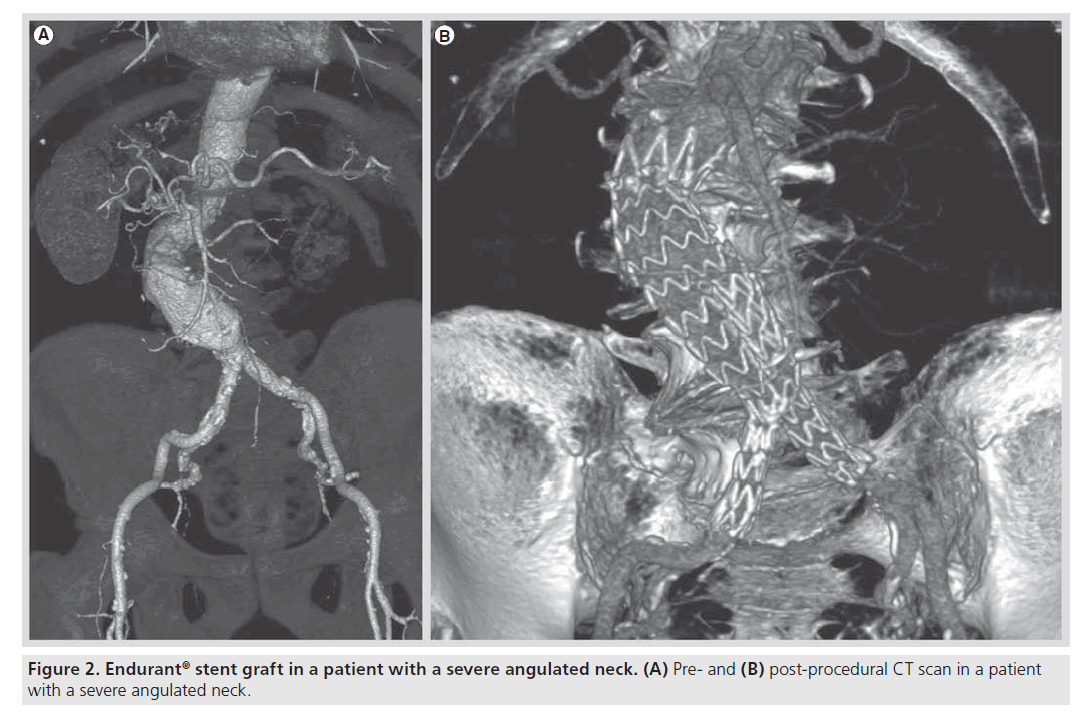
Recent studies have also demonstrated the feasibility of the Endurant stent graft in offlabel conditions (Figure 2) [19,20]. In particular, Bastos Gonçalves et al. demonstrated that the Endurant stent graft has similar early results in terms of Type I endoleak, major complication and survival in patients with or without severe proximal aneurysm neck angulation [20]. However, in our last published experience we demonstrated that the applicability of the Endurant stent graft in off-label conditions was good in terms of survival, morbidity or reinterventions, even if its use outside the IFU was responsible for a higher risk of Type I endoleak [20]. Therefore, a word of caution should be given regarding the application of EVAR outside the IFU, because in selected patients in those centers with a great experience in aortic endovascular procedures, it remains a risky procedure.
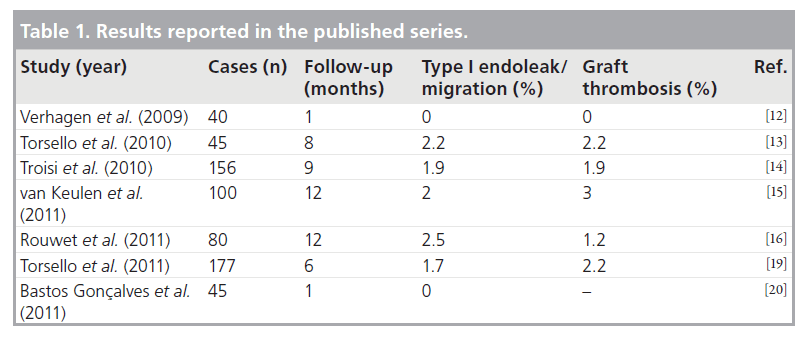
Finally, the use of low-profile endograft delivery systems seems to be associated with a lower rate of access-related complications [9]. Furthermore, in some published experiences the Endurant stent graft seems to reduce limb graft complications, increasing indications for EVAR also in cases with small/calcified/tortuous iliac vessels [14,15]. In particular, van Keulen et al. reported a rate of 3% of limb occlusion during a follow-up of 1 year [15]. Table 1 resumes the results of Endurant stent graft reported in the published series.
Conclusion
After assessing the literature, the authors feel that EVAR with Endurant stent graft is feasible and effective in patients with both normal and complex anatomy of the proximal aortic neck. However, the expansion of the indications for EVAR even in patients considered ‘off-label’ is responsible for a higher risk of Type I endoleak, although the early and mid-term results in terms of survival, major complications and reintervention rates were similar to those observed in patients operated on according to the indications of the manufacturer. This could also justify a cautious application of EVAR in patients outside device-specific IFU. Finally, further studies are needed to evaluate the long-term results of this new-generation device in normal and complex morphological situations.
Future perspective
Over the last few years, endovascular procedure volume has rapidly increased and endovascular procedures have become the first-line treatment for many vascular diseases [21]. Concomitantly, open vascular surgery is becoming more challenging; in particular, open repair for EVAR often requires suprarenal cross clamping, renal vein division, and management of associated iliac aneurismal and occlusive disease [22].
In such cases, adjunctive endovascular procedures (chimney technique and management of hypogastric involvement) or alternative, more complex endovascular procedures (fenestrated/ branched stent grafts) have been proposed [23,24]. These techniques have obvious advantages, but they are feasible only in selected centers, where skilled endovascular specialists have comprehensive experience with aortic procedures.
Young physicians in vascular training lack the experience required to successfully perform these kinds of procedures and they therefore require devices that are more easily utilized in complex cases. In this field, the Endurant stent graft represents an adjunctive weapon in the armamentarium of young vascular specialists.
In fact, the graft has a low-profile delivery system to overcome complex access vessels, an easy control–release deployment of the device, and a partial repositionability of the suprarenal stent to ensure an accurate sealing above the renal arteries, even in patients with challenging aneurysm neck.
The wide use of the Endurant stent graft in the clinical practice and its first encouraging results even in patients with complex aortic anatomy, empowered vascular surgeons to better treat patients with AAA. The Endurant is one example of the continuous development of endovascular technologies, which enable vascular surgeons to treat more complex vascular diseases. In the future, vascular surgeons’ competence will experience a further expansion in the field of endovascular procedures and hence lead to the establishment of a new-generation vascular specialist [25,26].
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪ New-generation devices are being developed with the aim of providing safe and effective alternative approaches to treating the unmet clinical need that clinicians face on a daily basis.
▪ The stent graft appears to be efficacious and safe for endovascular management of abdominal aortic aneurysms in patients with hostile anatomy of the proximal aortic neck and iliac arteries.
▪ The device is a valued alternative for vascular specialists in Europe, as well as all over the world.
Endurant stent graft
▪ The graft is a woven structure of multifilament polyester yarn supported by an electropolished nitinol stent structure.
▪ The suprarenal stent is laser cut with anchoring pins near the proximal end as a part of the nitinol structure.
▪ The wire-formed M-shaped body stents provide conformability and flexibility during the deployment.
▪ The graft is loaded inside a delivery system, ranging from 18 to 20 F for the main body and from 14 to 16 F for the iliac extensions.
▪ The delivery system features a tip-capture mechanism to ensure the accurate control–release deployment of suprarenal stents.
Clinical results
▪ Excellent outcomes both in the early (1.3% Type I endoleak rate) and mid-term periods (96.9% of freedom from Types I/III endoleak at 2 years).
▪ Similar early results in terms of Type I endoleak, major complication and survival in patients with or without severe proximal aneurysm neck angulation.
Future perspective
▪ Endurant stent graft represents an adjunctive weapon in the armamentarium of young vascular specialists.
▪ Vascular surgeons’ competence will experience a further expansion on the field of endovascular procedures.
References
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 5(6), 491–499 (1991).
- EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 365(9478), 2179–2186 (2005).
- EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 365(9478), 2187–2192 (2005).
- Lederle FA, Freischlag JA, Kyriakides TC, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA 302(14), 1535–1542 (2005).
- United Kingdom EVAR Trial Investigators. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N. Engl. J. Med. 362(20), 1872–1880 (2010).
- Hobo R, Kievit J, Leurs LJ et al. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: a EUROSTAR study. J. Endovasc. Ther. 14(1), 1–11 (2007).
- Lee JT, Lee J, Aziz I et al. Stent-graft migration following endovascular repair of aneurysms with large proximal necks: anatomical risk factors and long-term sequelae. J. Endovasc. Ther. 9(5), 652–664 (2002).
- Albertini JN, Perdikidess T, Soong CV et al. Endovascular repair of abdominal aortic aneurysms in patients with severe angulation of the proximal neck using a flexible stentgraft: European Multicenter Experience. J. Cardiovasc. Surg. 47(3), 245–250 (2006).
- Murray D, Ghosh J, Khwaja N et al. Access for endovascular aneurysm repair. J. Endovasc. Ther. 13(6), 754–761 (2006).
- Hinchliffe RJ, Ivancev K, Sonesson B et al. “Paving and cracking”: an endovascular technique to facilitate the introduction of aortic stent-grafts through stenosed iliac arteries. J. Endovasc. Ther. 14(5), 630–633 (2007).
- Criado FJ. Iliac arterial conduits for endovascular access: technical considerations. J. Endovasc. Ther. 14(3), 347–351 (2007).
- Verhagen HJ, Torsello G, de Vries JP et al. Endurant stent-graft system: preliminary report on an innovative treatment for challenging abdominal aortic aneurysm. J. Cardiovasc. Surg. 50(2), 153–158 (2009).
- Torsello G, Troisi N, Tessarek J et al. Endovascular aortic aneurysm repair with the Endurant stent-graft: early and 1-year results from a European multicenter experience. J. Vasc. Interv. Radiol. 21(1), 73–80 (2010).
- Troisi N, Torsello G, Donas KP et al. Endurant stent-graft: a 2-year, single center experience with a new commercially available device for the treatment of abdominal aortic aneurysms. J. Endovasc. Ther. 17(3), 439–448 (2010).
- van Keulen JW, de Vries JP, Dekker H et al. One-year multicenter results of 100 abdominal aortic aneurysm patients treated with the Endurant stent graft. J. Vasc. Surg. 54(3), 609–615 (2011).
- Rouwet EV, Torsello G, de Vries JP et al. Final results of the prospective European trial of the Endurant stent graft for endovascular abdominal aortic aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 42(4), 489–497 (2011).
- Böckler D, Fitridge R, Wolf Y et al. Rationale and design of the Endurant stent graft natural selection global postmarket registry (ENGAGE): interim analysis at 30 days of the first 180 patients enrolled. J. Cardiovasc. Surg. 51(4), 481–491 (2010).
- Makaroun MS, Tuchek M, Massop D et al. One year outcomes of the United States regulatory trial of the Endurant stent Graft System. J. Vasc. Surg. 54(3), 601–608 (2011).
- Torsello G, Troisi N, Donas KP et al. Evaluation of the Endurant stent graft under instruction for use vs. off-label conditions for endovascular aortic aneurysm repair. J. Vasc. Surg. 54(2), 300–306 (2011).
- Bastos Gonçalves F, de Vries JP, van Keulen JW et al. Severe proximal aneurysm neck angulation: early results using the Endurant stentgraft system. Eur. J. Vasc. Endovasc. Surg. 41(2), 193–200 (2011).
- Schanzer A, Steppacher R, Eslami M et al. Vascular surgery training trends from 2001–2007: a substantial increase in total procedure volume is driven by escalating endovascular procedure volume and stable open procedure volume. J. Vasc. Surg. 49(5), 1339–1344 (2009).
- Costin JA, Watson DR, Duff SB et al. Evaluation of the complexity of open abdominal aneurysm repair in the era of endovascular stent grafting. J. Vasc. Surg. 43(5), 915–920 (2006).
- Donas KP, Torsello G, Austermann M et al. Use of abdominal chimney grafts is feasible and safe: short-term results. J. Endovasc. Ther. 17(5), 589–593 (2010).
- Troisi N, Donas KP, Austermann M et al. Secondary procedures after aortic aneurysm repair with fenestrated and branched endografts. J. Endovasc. Ther. 18(2), 146–153 (2011).
- Nichols WK, Wei W. Has open vascular surgery disappeared? Mo. Med. 108(3), 182–186 (2011).
- Troisi N, Torsello G, Donas KP. Commentary: Endurant stent graft: a newgeneration device for a new generation of vascular specialist. J. Endovasc. Ther. 18(6), 786–788 (2011).