Review Article - Interventional Cardiology (2014) Volume 6, Issue 4
Cryoballoon ablation for atrial fibrillation
- Corresponding Author:
- Christian
Sticherling
Division of Cardiology, University Hospital Basel, Petersgaben 4, CH-4031 Basel, Switzerland
E-mail: christian.sticherling@usb.ch
Abstract
Atrial fibrillation is the most common cardiac arrhythmia occurring in 1–2% of the general population. It is associated with a reduction of functional status, quality of life and overall survival as well as with an increase in hospital admissions and stroke rate. Pulmonary vein (PV) isolation is the cornerstone of the interventional treatment of atrial fibrillation. Catheter ablation using radiofrequency (RF) energy has been shown to reduce symptoms, improve quality of life, and decrease hospitalizations. Cryoablation using a balloon catheter (Arctic Front Advance, Medtronic CryoCath) has emerged as an alternative to RF ablation (RFA) for achieving PV isolation. In this article we will review the biophysics of cryothermal tissue injury and the contemporary role of cryoablation in PV isolation. Also, anatomical predictors of successful cryoablation, complications and future perspectives will be discussedKeywords
atrial fibrillation, cardiac arrhythmia, cryoballoon ablation, pulmonary vein isolation, radiofrequency ablation
Atrial fibrillation is the most common cardiac arrhythmia occurring in 1–2% of the general population [1,2]. It is associated with a reduction of functional status, quality of life and overall survival as well as with an increase in hospital admissions and stroke rate [3]. Pulmonary vein (PV) isolation is the cornerstone of the interventional treatment of atrial fibrillation [4]. Catheter ablation using radiofrequency (RF) energy has been shown to reduce symptoms, improve quality of life, and decrease hospitalizations [5]. Cryoablation using a balloon catheter (Arctic Front Advance, Medtronic CryoCath) has emerged as an alternative to RF ablation (RFA) for achieving PV isolation. In this article we will review the biophysics of cryothermal tissue injury and the contemporary role of cryoablation in PV isolation. Also, anatomical predictors of successful cryoablation, complications and future perspectives will be discussed
Historical perspective
Since the first description of cryothermal myocardial lesions in 1948 [6] and the first application of cryoenergy on the cardiac conduction system in 1964 [7], cryoenergy ablation has evolved as a clinical tool for numerous indications. Initially, cryoablation was successfully used for the surgical treatment of accessory pathways, ventricular tachycardia, atrioventricular (AV) node re-entry tachycardia, and to ablate the AV node. Transcatheter cryoablation was first used in humans in 1998, for percutaneous transcatheter cryoablation of the AV node [8]. Since then, cryoablation technology improved and cryoablation is more widely used in cardiac electrophysiology for various clinical indications [9], particularly in the ablation of structures close to the AV node [10].
Principally, two different techniques can be used for PV isolation. Initially, point-bypoint ablation imitating the RF technique was advocated. It could be demonstrated that the PVs could be isolated with the same success rate as with RF but at the cost of significantly longer procedure and fluoroscopy times [11,12].
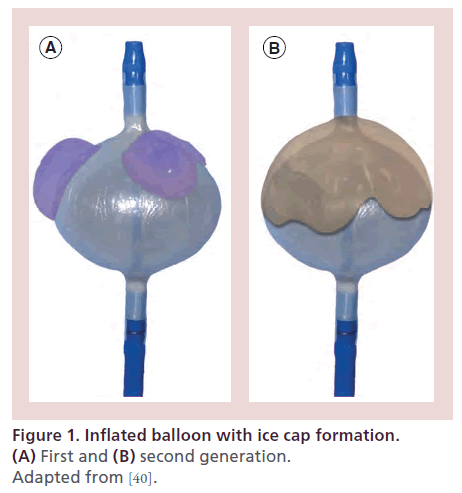
Therefore, research efforts resulted in an expandable cryoablation balloon catheter designed specifically for PV isolation. The deflectable, over the wire system contains an inner and outer balloon (Arctic Front Advance CryoAblation Catheter, Medtronic CryoCath). The catheter is 10.5 Fr and consists of an injection and exhaust lumen for nitrous oxide injection, a central lumen for guidewire or circular catheter (Achieve, Medtronic CryoCath) positioning and contrast injection, a dual pull wire mechanism and a thermocouple on the proximal end of the balloon. The currently available second-generation cryoablation catheter (Arctic Front Advance) uses the so-called ‘EvenCool Cryo Technology’ that distributes the coolant (nitrous oxide, N2O) more homogenously and thereby increases the effective surface area of the balloon (Figure 1). The balloon is available in two diameters (23 and 28 mm) and is introduced through a deflectable transseptal sheath with 12 Fr inner diameter.
Figure 1. Inflated balloon with ice cap formation. (A) First and (B) second generation. Adapted from [40].
Biology of cryoablation
Lesion formation with cryoablation relies on freezing and thawing the tissue in contact with the balloon or the catheter. Freezing starts at the catheter–tissue interface and then radially spreads into the tissue. Mechanisms of lesion formation and tissue injury can be divided in three phases: the freeze–thaw phase, the hemorrhage and inflammation phase, and the fibrosis phase [13]. In the freeze–thaw phase, hypothermia causes cardiac myocytes to become rigid, ion pumps lose their transport capabilities and the intracellular pH decreases [14]. Whether these changes are transient is determined by the minimal temperature and duration of cryoenergy application. Progressive freezing results in formation of ice crystals, first in the extracellular matrix and then intracellulary. Intracellular crystals cause distortion of intracellular organelles and extracellular crystals render the extracellular space hypertonic which, in turn, results in cellular shrinkage and causes the intracellular space to become hyperosmotic and acidic. This results in protein damage, enzyme function impairment and damage to the cell membrane and cell organelles. Freezing also causes changes in the microcirculation with hyperemia, interstitial edema and microthrombosis which ultimately causes tissue ischemia. After cessation of the freezing cycle, the tissue returns to body temperature, which is called the thawing phase. At this point, vascular hyperemia occurs. In the second phase of hemorrhage and inflammation, edema occurs and fluid transverses endothelial cells resulting in coagulation necrosis. After weeks, in the fibrosis phase, fibrosis and apoptosis of the frozen tissue define the cryoablation lesion [15].
Potential clinical advantages & disadvantages of cryoablation
Due to the mechanism of cryothermal lesion formation, cryocatheters (both focal and balloon-based) adhere to the tissue and exhibit greater stability during the ablation procedure. This minimizes catheter movements caused by the beating heart and respiration which may be advantageous when ablating in delicate positions. Due to the potential reversibility of the early lesion some advocate the use of cryoablation in critical locations like in para-Hissian accessory pathways [16].
Apart from this specific indication, catheter-based point-to-point cryoablation has not become clinical routine since it is too time consuming due to the long freezing/thawing cycles [17]. Cryoablation lesions result in minimal endothelial disruption and may therefore carry a lower risk for thrombus formation [18,19]. In addition, it appears to activate platelets and coagulation factors to a lesser degree than RFA. All these lead to a potentially lower risk of thromboembolism and stroke [20]. Well-defined lesion formation with cryoablation and less tissue disruption compared with RF lesions could lead to fewer acute and long-term complications. These include a potentially lower risk of cardiac perforation and esophageal injury in the short term [14] and a decreased risk for PV stenosis in the long term [21,11]. However, large-scale comparisons to prove this are lacking. It appears that cryoablation is associated with less pain and discomfort during ablation compared with RFA, but does not obviate the need for some sort of conscious sedation [22]. Finally, some non-randomized trials have shown potentially reduced procedural times with cryoballoon ablation (CBA), whereas focal pointby- point cryoablations may be associated with longer procedure times [23].
Potential disadvantages of CBA include the need for larger transseptal sheath which potentially results in less maneuverability and could translate in a higher incidence of vascular access complications or tamponade. However, incidence in both vascular access site complications and tamponade (1.79 and 1.4%) are comparable to those with RFA (1.47 and 1.31) [24,4].
Also, CBA is of limited use in patients with persistent, especially long-standing persistent atrial fibrillation (AF) where additional left atrial substrate modification may be required. In these situations, in addition to CBA, additional point-by-point ablation catheter has to be used [25]. Box 1 shows advantages and disadvantages of CBA.
Practical considerations
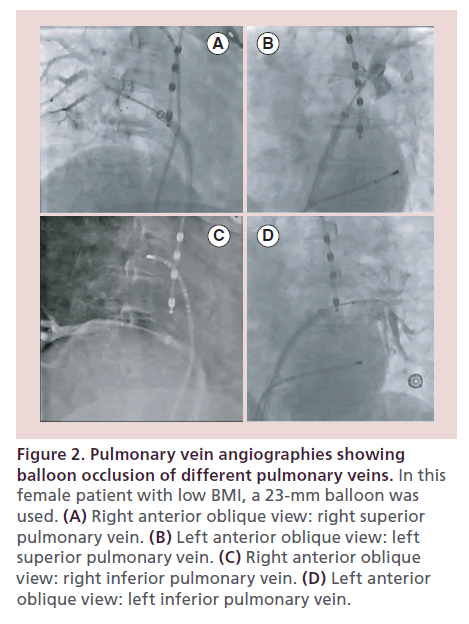
For CBA, a femoral vein access for two sheaths is commonly employed. One short sheath is inserted for a second catheter used as a reference and for pacing. This catheter can later position in the superior vena cava (SVC) for phrenic nerve (PN) stimulation during the ablation of the right-sided veins. A single transseptal puncture is performed for access to the left atrium with the use of a standard transseptal sheath. The transseptal sheath is exchanged for a 12-Fr deflectable delivery sheath (15-Fr outer diameter; FlexCath, Medtronic, MN, USA) in an over-the-wire technique, with the J-tip 0.032 inch wire placed in a left PV. Full heparinization with a target activated clotting time of 350 s is maintained throughout the procedure. After that, the balloon is advanced over the spiral mapping catheter (SMC; Achieve, Medtronic, MN, USA) to the left atrium. With the SMC, PVs are cannulated and PV signals are recorded. We usually start with left-sided PVs, from superior to inferior. Once the SMC catheter is positioned in the selected PV, the balloon is inflated in the cavity of the left atrium. After inflation, the balloon is advanced to the PV ostium and contrast injection is performed through the central lumen of the catheter. Now complete occlusion of the PV is documented (Figure 2). Inability to completely seal the PV will result in convective heating of the tissue from circulating blood which reduces lesion formation. While the complete occlusion of the superior veins is usually straight forward, the inferior, and in particular the right inferior PV (RIPV) may prove to be challenging. The easiest way to achieve better balloon–tissue contact is to support the catheter by advancing the sheath, use of catheter rotation, catheter/sheath deflection or repositioning of the SMC to another PV branch. For cases in which these maneuvers do not suffice, several methods have been described. The ‘hockey-stick’ technique has been described with early branching RIPV and the ‘pull-down’ technique has been described for inferior veins. With the hockey-stick technique, the guide wire or SMC is advanced in an early branch of the inferior vein and the sheath is advanced with maximal bend which provides support for additional balloon pressure inferiorly. The pull-down technique is another option when it is difficult to achieve good tissue contact with the inferior circumference of the vein. The SMC and the balloon are advanced and if contact is present at the superior circumference of the ostium, the freezing cycle is started. When the balloon attaches to the superior PV circumference during the freeze the whole system is pulled down to close the gap inferiorly. [26]. Although the optimal occlusion of the PVs is a goal, it may not be necessary in all cases using the second-generation balloon since the balloon increases from 26.5 to 28 mm during the energy delivery [27]. This increase in diameter facilitates occlusion during CBA even if the preablation angiogram did not show complete occlusion.
Figure 2. Pulmonary vein angiographies showing balloon occlusion of different pulmonary veins. In this female patient with low BMI, a 23-mm balloon was used. (A) Right anterior oblique view: right superior pulmonary vein. (B) Left anterior oblique view: left superior pulmonary vein. (C) Right anterior oblique view: right inferior pulmonary vein. (D) Left anterior oblique view: left inferior pulmonary vein.
After adequate occlusion is verified, cryoablation is performed. With the second-generation CB, two freezing cycles of 240 s in each vein with constant monitoring of PV signals on the SMC catheter are commonly performed. During applications at the right PVs, the coronary sinus catheter is placed in the SVC for PN stimulation during the ablation. There is accumulating evidence that the use of the second-generation CB may allow for only one 180-s freeze per vein for successful PV isolation (PVI) [28].
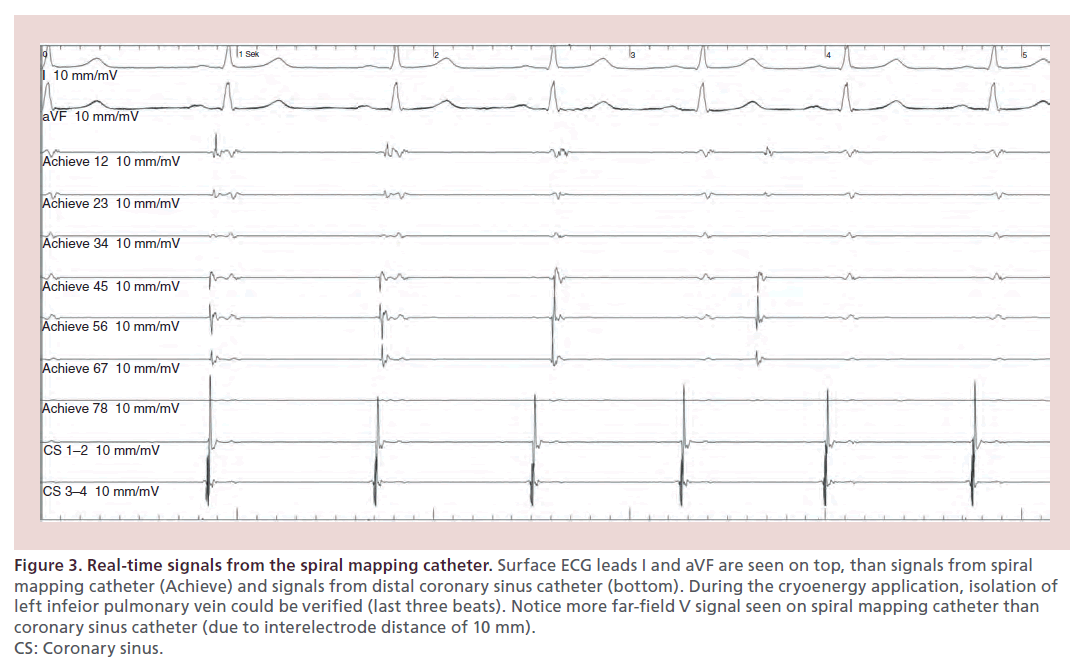
There is evidence that in patients with documented PV potentials on the SMC during the ablation, time to isolation is associated with sustained PVI success [29]. Due to the design of the catheter tip (‘long nose’), ostial recordings are often not possible during the ablation [30]. Therefore, in practice, PV recordings can only be seen in approximately 50% of the cases. Furthermore, the interpretation of the signals differs from those retrieved from a conventional circumferential mapping catheter (Figure 3).
Figure 3. Real-time signals from the spiral mapping catheter. Surface ECG leads I and aVF are seen on top, than signals from spiral mapping catheter (Achieve) and signals from distal coronary sinus catheter (bottom). During the cryoenergy application, isolation of left infeior pulmonary vein could be verified (last three beats). Notice more far-field V signal seen on spiral mapping catheter than coronary sinus catheter (due to interelectrode distance of 10 mm). CS: Coronary sinus.
In one study with 141 patients, PV recordings were possible in 235/568 PVs (41%). The median time to isolation was significantly shorter in patients without reconnection (39 vs 125 s; p < 0.001). Time to isolation of less than 83 s was identified as a predictor of no reconnection (86% sensitivity and 97% specificity). Another predictor of success is the lowest achieved CB temperature [31]. Fürnkranz et al. reported that a temperature less than -51°C at the end of a freeze had a specificity of 100% for successful PVI in all PVs. On the other hand, after 120 s of freezing, CB temperature greater than or equal to -36°C predicted an ineffective freeze with a 97% specificity (positive predictive value: 82%) in the superior PVs and a temperature greater than or equal to -33°C predicted an ineffective freeze with 95% specificity (positive predictive value: 80%) in the inferior PVs. How these results can be transferred one to one to the second-generation CB still has to been investigated.
CBA efficacy & safety
Success rate
In 2008, Neumann and colleagues published the first prospective study using CBA [32]. They included 346 patients with paroxysmal (n = 293) and persistent (n = 53) AF. Acute isolation of PVs with the CB technique could be achieved in 92.5% of 1403 PVs and in 97% with additional ‘touch-up’ lesions with a focal cryocatheter. Sinus rhythm was maintained in 74% of patients with paroxysmal AF and 42% of patients with persistent AF with a median follow-up of 12 months. These results were similar to other published studies, although with a shorter follow-up [33].
The STOP AF trial was the first randomized controlled, multicenter trial comparing CBA to antiarrhythmic drug therapy and was published in 2013 [34]. The trial included 245 patients with paroxysmal AF randomized to CBA (n = 163) or medical therapy (n = 82). At 12-month follow-up, freedom from AF recurrence was achieved in 69.9% of patients in the cryoablation arm compared with 7.3% in the medical therapy group. Of note, follow-up in this trial included telephonic monitoring every week and Holter ECG monitoring at 1, 3, 6, 9 and 12-month follow-up. This pivotal trial led to US FDA approval of the CB use for AF ablation in the USA [35].
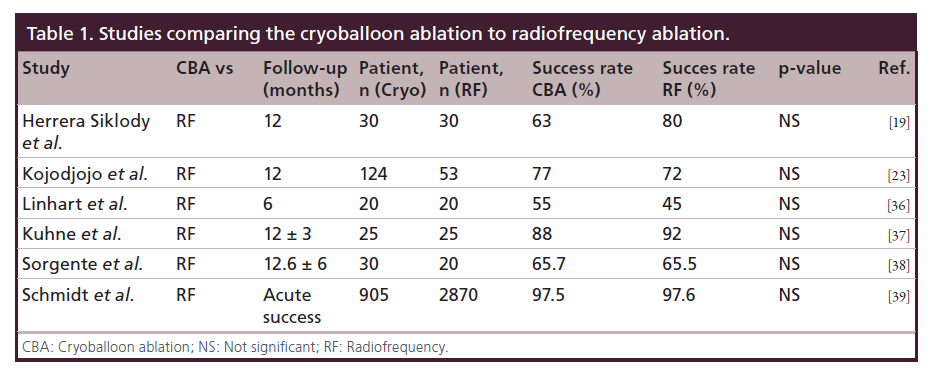
In recent years, results from several studies comparing RFA to CBA were reported, but none of these studies was performed in a randomized controlled fashion (Table 1) [19,23,36–39]. The largest number of patients was included in the German Ablation Registry which prospectively included patients undergoing ablation for paroxysmal AF. A total of 3775 patients were enrolled of which 905 underwent CBA and 2870 RFA. The acute success rates in both groups were similar (97.5% CBA group vs 97.6% in RF group; p = 0.81). Procedure times were similar but ablation and fluoroscopy times were higher in CBA group. However, the value of the results of this large registry is very limited due to the lack of reported follow-up data.
Current knowledge indicates that RFA and CBA perform comparably. Although results are similar, a direct comparison of these two methods has not been performed in a randomized controlled fashion. The ongoing randomized ‘Fire and Ice’ trial (NCT01490814) is expected to be completed in 2015.
Recently, second-generation CB became available and is widely used for PVI. The new balloon has significantly greater cooling area with more powerful and homogenous cooling behavior in vitro as shown in a study by our group [40]. Also, a report by Bordignon et al. [41] showed higher biomarker release with secondgeneration CB than with the first-generation CB assuming better lesion formation with second-generation CB. Higher biomarker release was associated with better outcomes after 6 months. Several single-center studies compared efficacy of second- versus first-generation CB and have shown higher procedural efficacy, shorter procedure and fluoroscopy duration [42,27]. Whether better cooling characteristics, acute and short term success, translate in better long-term outcomes will have to be evaluated in further studies.
Complications
Periprocedural complications with CBA have been reported in 3–5% [24] of cases, which is comparable to complication rates with RFA [4,43]. The most common complication of CBA is right PN palsy (PNP). In a review by Andrade et al. [24] PNP occurred in 86 out of 1349 procedures (6%) with the use of the first-generation balloon. Most of the PNPs recovered spontaneously during 1-year follow-up, with only 0.4% persisting after 1 year. PNP occurred more often with the use of the 23-mm balloon and is mostly described during the right superior PV (RSPV) ablation, although it can also occur during ablation at the RIPV [44]. In most individuals, the PN runs much closer to the RSPV than to the RIPV [45]. Recently published data show differences in reported PNPs using the second-generation balloon for CBA. A study by Casado-Arroyo et al. reported a high rate of PNP of 19.5% (8/41) with the second-generation 28-mm balloon [44]. The incidence was higher with RSPV ablation, but also occurred during RIPV ablation. Only one patient (2.5%) had persistent PNP after 7-month follow-up. In contrast, the study by Metzner et al. [46] reported an incidence of 3.5% (4/115) of PNP while ablating the RSPV. There are several methods for functional PN monitoring. At our institution, the deflectable coronary sinus catheter is positioned in the SVC cranially to the balloon. Subsequently, high output pacing (12V/2.9 ms at cycle length 1000 ms) is performed and diaphragmatic capture monitored by palpation [47]. In case of loss of capture (or a decrease in diaphragmatic activity) the freezing cycle should be stopped immediately. Another option is monitoring of the compound motor action potential (CMAP) [48,49]. For that purpose, the standard surface electrode from the right arm is placed at the sternum 5 cm above the xyphoid process and from the left arm along the right lower rib cage 16 cm apart. In this configuration a large amplitude signal can be recorded in lead I during PN capture [49]. A decrease in amplitude of >30% may predict PNP and mandates interruption of cryoablation. Recently, a novel modified technique for recording CMAP was described with recording CMAP from quadripolar catheter positioned in a subdiaphragmatic hepatic vein [50]. In this study, cryoablation was terminated when the CMAP decreased by more than 30%. No PNPs were reported.
PV stenosis reported with CBA ranges from 0.17% to 3% depending on method being used for diagnosing and defining PV stenosis [24]. Incidence of symptomatic PV stenosis or PV stenosis requiring treatment was 0.17% [24]. In the STOP AF trial, PV stenosis was reported in 3% of patients (7/228 patients) [34]. The differences in reported incidence might be due to the different criteria used to define PV stenosis and methods used to access PV stenosis between reports. In STOP AF, 75% reduction of cross-sectional area (which approximately corresponds to 50% reduction in diameter) was used as definition of PV stenosis [34].
Periprocedural thromboembolic complications during CBA, mainly stroke and transient ischemic attack, are reported in 0.3–0.42% [24], which is comparable to 0.3–0.9% of RFA [4].
Furthermore, with the use of the more potent second- generation balloon, affections of the esophageal mucosa and even cases of atrioesophageal fistula have been reported [51,52]. Incidence of esophageal lesions or atrioesophageal fistulas may be difficult to compare since it is very infrequent in both CBA and RFA patients and would require large databases to compare these complications.
Indications for cryoablation & patient selection
Several factors have to be taken into account when choosing a patient for CBA PVI. First, one has to bear in mind that in terms of success rates and outcome measure, no difference to conventional RF could be shown. This also applies for complication rates with the exception of a potentially higher incidence of atrioesophageal fistula in patients undergoing RFA and PNP in patients undergoing CBA. In both cases, methods have been developed to decrease the incidence of these complications Until these two methods are compared head to head, patient selection and indications for CBA (or RFA) cannot be performed on the basis of outcome data and are subject to a operator preference.
Second, the type of AF, respectively the planned lesions set, matters. CBA was dominantly shown to be successful, safe and feasible in patients with paroxysmal AF. In a review by Andrade et al. [24], three studies which included a total of 84 patients with persistent AF, 1-year freedom from AF was 45.2% [32,23]. Mansour et al. [25]. combined CBA and RFA in 22 patients with persistent AF. In 19 patients (86%) after CBA, complex fractionated atrial electrograms were targeted using the RF catheter and in 10 patients (45%) lines were drawn using RF catheter. Using this combined approach, freedom from AF at 6 months was 86.4%. Recently, centers including patients with persistent AF [53,54] had similar 1-year success rates of 49 and 50%. It seems that in patients with long-standing persistent AF, however, additional focal or linear ablations are needed [55,56], which cannot be achieved using CBA only. In patients with paroxysmal AF, after a failed ablation with first-generation CB, CBA with second-generation CB could be considered based on recent reports of improved procedural efficacy [27,42].
Anatomical predictors could be used for patient selection. Our group described anatomical characteristics which influence cryoenergy transfer and can be used as predictors of acute and mid-term success for CBA using the 28-mm balloon [57]. For the left-sided veins, a continuous sharp left lateral ridge between the PVs and the left lateral appendage and a sharp carina between the left superior and left inferior PV predicted acute and mid-term failure. For the RIPV a nonperpendicular angle between the axis of the PV and the ostial plane and an early branching PV with change in the axis angle were identified as predictors of acute and mid-term failure. However, these results are based on procedures using the first-generation CB because the second-generation CB was not available at the time. Finally, a clinical risk score (BASE AF2) has been proposed for outcome prediction and patient selection for CBA [58]. The score consists of BMI >28 kg/m (1), atrial dilatation >40 mm (1), current smoking (1), early AF recurrence (1), duration of AF history >6 years (1) and nonparoxysmal type (1). A score of ≥3 predicted AF recurrence with sensitivity of 80.8% and a specificity of 91.6%. Although interesting, this score needs to be further evaluated in prospective trials and larger number of patients.
Reimbursement may also affect patient selection. Recently, a cost comparison between the different methods was published [59]. In the report, both lowest and highest calculated costs for CBA were considerably higher than for RFA.
Finally, the best evidence for use of CBA exists for patients with paroxysmal AF and of the decision to use CBA should depend on the availability, operator experience as well as anatomical and clinical parameters described above.
Conclusion & future perspective
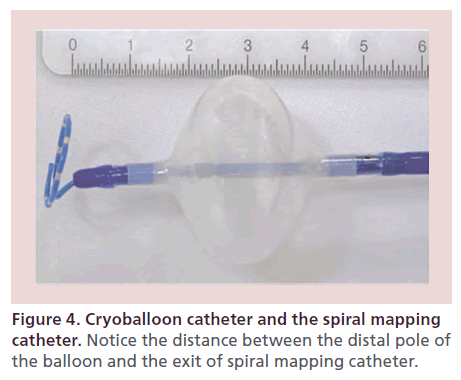
With the use of the SMC catheter, PV potentials can be recorded during CBA in real time. This can help to monitor PV disconnection during CBA, titrate duration of cryoenergy application and also reduce the need for an additional circular mapping catheter for verification of PVI. Furthermore, the use of the SMC catheter has been associated with shorter procedure and fluoroscopy times [60]. In a report by Kühne et al., real-time recordings of PVs could be obtained only in 50% of PVs, with a somewhat higher rate with leftsided veins [30]. One of the potential reasons is the distance of 17 mm between the tip of the CB catheter and distal balloon pole (Figure 4). Recently, real-time recordings and proof of left atrial–PV disconnection was obtained successfully in almost all PVs using standard SMC positioning and additional torque maneuvers [61]. Although promising, there is still potential for further improvement of the SMC catheter for instance by shortening the distance between the catheter tip and the balloon tip [30,61] or by having variable catheter sizes [61].
Also, with rising experience with CBA, the vast majority of veins can be isolated with the help of the described maneuvers [26]; further improvements in catheter and sheath technology will likely further improve the success rate of CBA in the future.
Financial & competing interests disclosure
N Pavlović was supported by an Education Grant of the European Heart Rhythm Association. He received lecture fees from Abbott, Pliva/Teva Pharmaceuticals, Sandoz. M Kühne has served on the speakers’ bureau for Boston Scientific, St Jude Medical, Biotronik and Medtronic and serves as a proctor for Medtronic for CBA. He has received consulting fees from Boehringer Ingelheim, Bayer, MSD and Novartis. He has received lecture fees from Biotronik, Boston Scientific, SJM, Sanofi Aventis, Sorin, Bayer, Böhringer Ingelheim. C Sticherling has served on the speakers’ bureau for Boston Scientific, Biotronik, Sorin and Medtronic and serves as a proctor for Medtronic for CBA. He has received consulting fees from Boehringer Ingelheim, Sorin, Medtronic and Biotronik. He has received lecture fees from Biotronik, Sanofi Aventis, Sorin, Bayer and Böhringer Ingelheim. Both S Knecht and T Reichlin received no financial assistance. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
History
• Cryoenergy ablation was developed as an alternative to radiofrequency ablation (RFA).
• It is used for ablation of wide range of arrhythmias, especially in children and in the proximity of the atrioventricular node.
• Cryoballoon ablation (CBA) emerged as a potential ‘single shot’ tool for pulmonary vein isolation.
Biology of cryothermal tissue injury
• Lesion formation develops in three phases with cryoablation: freeze–thaw phase, the hemorrhage and inflammation phase, and the fibrosis phase.
Potential clinical advantages
• Need for single transseptal puncture.
• Shorter procedural times.
• No need for electroanatomical mapping systems.
• Less need for analgesia/sedation.
Potential clinical disadvantages
• Limited value when left atrial substrate modification is needed (persistent atrial fibrillation).
• Longer fluoroscopy times.
• Need for contrast media.
Cryoballoon success rates
• Acute and long-term success rates comparable to RFA.
• RFA and CBA have not been compared in a randomized manner.
• Second-generation cryoballoon has better cooling profile than first-generation balloon.
• Acute and short-term success rates are better with second-generation cryoballoon.
• Randomized controlled trial is being conducted to compare RFA and CBA.
Cryoballoon ablation safety
• Complication rates with CBA are similar to RFA.
• Phrenic nerve palsy occurs more often with CBA and is the most often complication with CBA.
• PNP occures more often with right superior pulmonary vein ablation, although it has been reported with right inferior pulmonary vein ablation also.
• Different methods of PNP prevention have been described.
• Most often used is high output pacing in superior vena cava proxymal to CBA and manual or fluoroscopic monitoring of diaphragmatic excursions.
Patient selection
• Strongest evidence exists for patients with paroxysmal atrial fibrillation.
• Patient selection should depend on cryoballoon availability, operator preference/experience and clinical and anatomical predictors.
• Clinical and anatomical predictors seem to be less important with second-generation cryoballoon.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Camm AJ, Lip GYH, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 33(21), 2719–2747 (2012).
- Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86(5), 516–521 (2001).
- Stewart S, Hart CL, Hole DJ, McMurray JJV. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am. J. Med. 113(5), 359–364 (2002).
- Cappato R, Calkins H, Chen S-A et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3(1), 32–38 (2010).
- Bunch TJ, May HT, Bair TL et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm 10(9), 1272–1277 (2013).
- Hass GM, Taylor CB. A quantitative hypothermal method for production of local injury to tissue. Proc. Annu. Meet. Cent. Soc. Clin. Res. US 20, 49 (1947).
- Lister JW, Hoffman BF, Kavaler F. Reversible cold block of the specialized cardiac tissues of the unanaesthetized dog. Science 145(3633), 723–725 (1964).
- Dubuc M, Khairy P, Rodriguez-Santiago A et al. Catheter cryoablation of the atrioventricular node in patients with atrial fibrillation: a novel technology for ablation of cardiac arrhythmias. J. Cardiovasc. Electrophysiol. 12(4), 439–444 (2001).
- Friedman PL, Dubuc M, Green MS et al. Catheter cryoablation of supraventricular tachycardia: results of the multicenter prospective ‘frosty’ trial. Heart Rhythm 1(2), 129–138 (2004).
- Rodriguez L-M, Geller JC, Tse H-F et al. Acute results of transvenous cryoablation of supraventricular tachycardia (atrial fibrillation, atrial flutter, Wolff–Parkinson–White syndrome, atrioventricular nodal re-entr tachycardia). J. Cardiovasc. Electrophysiol. 13(11), 1082–1089 (2002).
- Tse H-F, Reek S, Timmermans C et al. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J. Am. Coll. Cardiol. 42(4), 752–758 (2003).
- Kettering K, Al-Ghobainy R, Wehrmann M, Vonthein R, Mewis C. Atrial linear lesions: feasibility using cryoablation. Pacing Clin. Electrophysiol. 29(3), 283–289 (2006).
- Lustgarten DL, Keane D, Ruskin J. Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog. Cardiovasc. Dis. 41(6), 481–498 (1999).
- Khairy P, Dubuc M. Transcatheter cryoablation part I: preclinical experience. Pacing Clin. Electrophysiol. 31(1), 112–120 (2008).
- Mazur P. Cryobiology: the freezing of biological systems. Science 168(3934), 939–949 (1970).
- Gaita F, Haissaguerre M, Giustetto C et al. Safety and efficacy of cryoablation of accessory pathways adjacent to the normal conduction system. J. Cardiovasc. Electrophysiol. 14(8), 825–829 (2003).
- Kuniss M, Vogtmann T, Ventura R et al. Prospective randomized comparison of durability of bidirectional conduction block in the cavotricuspid isthmus in patients after ablation of common atrial flutter using cryothermy and radiofrequency energy: the CRYOTIP study. Heart Rhythm 6(12), 1699–1705 (2009).
- Khairy P, Chauvet P, Lehmann J et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation 107(15), 2045–2050 (2003).
- Herrera Siklódy C, Arentz T, Minners J et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: a randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm 9(2), 189–196 (2012).
- Hochholzer W, Schlittenhardt D, Arentz T et al. Platelet activation and myocardial necrosis in patients undergoing radiofrequency and cryoablation of isthmus-dependent atrial flutter. Europace 9(7), 490–495 (2007).
- Feld GK, Yao B, Reu G, Kudaravalli R. Acute and chronic effects of cryoablation of the pulmonary veins in the dog as a potential treatment for focal atrial fibrillation. J. Interv. Card. Electrophysiol. 8(2), 135–140 (2003).
- Timmermans C, Ayers GM, Crijns HJGM, Rodriguez L-M. Randomized study comparing radiofrequency ablation with cryoablation for the treatment of atrial flutter with emphasis on pain perception. Circulation 107(9), 1250–1252 (2003).
- Kojodjojo P, O’Neill MD, Lim PB et al. Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart 96(17), 1379–1384 (2010).
- Andrade JG, Khairy P, Guerra PG et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm 8(9), 1444–1451 (2011).
- Mansour M, Forleo GB, Pappalardo A et al. Combined use of cryoballoon and focal open-irrigation radiofrequency ablation for treatment of persistent atrial fibrillation: results from a pilot study. Heart Rhythm 7(4), 452–458 (2010).
- Chun K-RJ, Schmidt B, Metzner A et al. The ‘single big cryoballoon’ technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur. Heart J. 30(6), 699–709 (2009).
- Fürnkranz A, Bordignon S, Schmidt B et al. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J. Cardiovasc. Electrophysiol. 24(5), 492–497 (2013).
- Chierchia G-B, Di Giovanni G, Sieira-Moret J et al. Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. J. Interv. Card. Electrophysiol. 39(2), 145–151 (2013).
- Dorwarth U, Schmidt M, Wankerl M, Krieg J, Straube F, Hoffmann E. Pulmonary vein electrophysiology during cryoballoon ablation as a predictor for procedural success. J. Interv. Card. Electrophysiol. 32(3), 205–211 (2011).
- Kühne M, Knecht S, Altmann D et al. Validation of a novel spiral mapping catheter for real-time recordings from the pulmonary veins during cryoballoon ablation of atrial fibrillation. Heart Rhythm 10(2), 241–246 (2013).
- Fürnkranz A, Köster I, Chun KRJ et al. Cryoballoon temperature predicts acute pulmonary vein isolation. Heart Rhythm 8(6), 821–825 (2011).
- Neumann T, Vogt J, Schumacher B et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J. Am. Coll. Cardiol. 52(4), 273–278 (2008).
- Van Belle Y, Janse P, Rivero-Ayerza MJ et al. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur. Heart J. 28(18), 2231–2237 (2007).
- Packer DL, Kowal RC, Wheelan KR et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 61(16), 1713–1723 (2013).
- Fitzgerald DM. Catheter ablation of atrial fibrillation: to freeze, or not to freeze, that is the question. J. Cardiovasc. Electrophysiol.25 (1), 8–10 2013).
- Linhart M, Bellmann B, Mittmann-Braun E et al. Comparison of cryoballoon and radiofrequency ablation of pulmonary veins in 40 patients with paroxysmal atrial fibrillation: a case-control study. J. Cardiovasc. Electrophysiol. 20(12), 1343–1348 (2009).
- Kühne M, Suter Y, Altmann D et al. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm 7(12), 1770–1776 (2010).
- Sorgente A, Chierchia GB, Capulzini L et al. Atrial fibrillation ablation: a single center comparison between remote magnetic navigation, cryoballoon and conventional manual pulmonary vein isolation. Indian Pacing Electrophysiol. J. 10(11), 486–495 (2010).
- Schmidt M, Dorwarth U, Andresen D et al. Cryoballoon versus RF ablation in paroxysmal atrial fibrillation: results from the German Ablation Registry. J. Cardiovasc. Electrophysiol. 25(1), 1–7 (2014).
- Knecht S, Kühne M, Osswald S, Sticherling C. Quantitative assessment of a second-generation cryoballoon ablation catheter with new cooling technology – a perspective on potential implications on outcome. J. Interv. Card. Electrophysiol. 40(1), 17–21 (2014).
- Bordignon S, Fürnkranz A, Dugo D et al. Improved lesion formation using the novel 28 mm cryoballoon in atrial fibrillation ablation: analysis of biomarker release. Europace 1(7), 987-993 (2014).
- Martins RP, Hamon D, Césari O et al. Safety and efficacy of a second-generation cryoballoon in the ablation of paroxysmal atrial fibrillation. Heart Rhythm 11(3), 386–393 (2014).
- Dagres N, Hindricks G, Kottkamp H et al. Complications of atrial fibrillation ablation in a high-volume center in 1,000 procedures: still cause for concern? J. Cardiovasc. Electrophysiol. 20(9), 1014–1019 (2009).
- Casado-Arroyo R, Chierchia G-B, Conte G et al. Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: a comparison between the first- and second-generation balloon. Heart Rhythm 10(9), 1318–1324 (2013).
- Sánchez-Quintana D, Cabrera JA, Climent V, Farré J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J. Cardiovasc. Electrophysiol. 16(3), 309–313 (2005).
- Metzner A, Rausch P, Lemes C et al. The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J. Cardiovasc. Electrophysiol. 25(5), 466–470 (2014).
- Kühne M, Knecht S, Altmann D et al. Phrenic nerve palsy during ablation of atrial fibrillation using a 28-mm cryoballoon catheter: predictors and prevention. J. Interv. Card. Electrophysiol. 36(1), 47–54 (2013).
- Franceschi F, Dubuc M, Guerra PG et al. Diaphragmatic electromyography during cryoballoon ablation: a novel concept in the prevention of phrenic nerve palsy. Heart Rhythm 8(6), 885–891 (2011).
- Franceschi F, Dubuc M, Guerra PG, Khairy P. Phrenic nerve monitoring with diaphragmatic electromyography during cryoballoon ablation for atrial fibrillation: the first human application. Heart Rhythm 8(7), 1068–1071 (2011).
- Franceschi F, Koutbi L, Mancini J, Attarian S, Prevôt S, Deharo J-C. Novel electromyographic monitoring technique for prevention of right phrenic nerve palsy during cryoballoon ablation. Circ. Arrhythm. Electrophysiol. 6(6), 1109–1114 (2013).
- Lim HW, Cogert GA, Cameron CS, Cheng VY, Sandler DA. Atrioesophageal fistula during cryoballoon ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. (2013).
- Stöckigt F, Schrickel JW, Andrié R, Lickfett L. Atrioesophageal fistula after cryoballoon pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 23(11), 1254–1257 (2012).
- Ferrero-de Loma-Osorio A, Izquierdo-de Francisco M, Martínez-Brotons A et al. Medium-term results of cryoballoon ablation of the pulmonary veins in patients with paroxysmal and persistent atrial fibrillation. First experience of a Spanish center. J. Interv. Card. Electrophysiol. 37(2), 189–196 (2013).
- Aytemir K, Oto A, Canpolat U et al. Immediate and medium-term outcomes of cryoballoon-based pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation: single-centre experience. J. Interv. Card. Electrophysiol. 38(3), 187–195 (2013).
- Verma A, Mantovan R, Macle L et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur. Heart J. 31(11), 1344–1356 (2010).
- Wu S-H, Jiang W-F, Gu J et al. Benefits and risks of additional ablation of complex fractionated atrial electrograms for patients with atrial fibrillation: a systematic review and meta-analysis. Int. J. Cardiol. 169(1), 35–43 (2013).
- Knecht S, Kühne M, Altmann D et al. Anatomical predictors for acute and mid-term success of cryoballoon ablation of atrial fibrillation using the 28 mm balloon. J. Cardiovasc. Electrophysiol. 24(2), 132–138 (2013).
- Canpolat U, Aytemir K, Yorgun H, Şahiner L, Kaya EB, Oto A. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish Cryoablation Registry. Int. J. Cardiol. 169(3), 201–206 (2013).
- Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Physician-controlled costs: the choice of equipment used for atrial fibrillation ablation. J. Interv. Card. Electrophysiol. 36(2), 157–165 (2013).
- Peyrol M, Sbragia P, Quatre A et al. Reduction of procedure duration and radiation exposure with a dedicated inner lumen mapping catheter during pulmonary vein cryoablation. Pacing Clin. Electrophysiol. 36(1), 24–30 (2013).
- Boveda S, Providência R, Albenque J-P et al. Real-time assessment of pulmonary vein disconnection during cryoablation of atrial fibrillation: can it be ‘achieved’ in almost all cases? Europace 16(6), 826–833 (2013).
• Description of different techniques for cannulating pulmonary veins with cryoballoon catheter.
•• Study showing that 3-min freezes might be sufficient for pulmonary vein isolation using the second-generation cryoballoon.
• Validation of spiral mapping catheter for real-time recordings during the cryoballoon ablation.
•• First prospective trial of cryoballoon ablation that included 346 patients undergoing atrial fibrillation ablation.
•• First randomized controlled trial comparing cryoballoon ablation and antiarrhythmic drug therapy in paroxysmal atrial fibrillation.