Research Article - Clinical Investigation (2022) Volume 12, Issue 12
Conception and clinical efficacy of a novel polymeric ashtma prevention treatment compared to Salbutamol
Abstract
Objectives: Asthma is becoming one of the fastest growing epidemics worldwide, affecting already nearly 9% of the population. This is a multifactorial, immunological disease, with a complex physiopathology involving the contact between asthma-triggering factors and nasal mucosa, nasal mucosa damage, the release of disease-specific TSLP, and other proinflammatory cytokines such as IL-5, 6, 25, 33, responsible for persistent upper respiratory tract inflammation. An effective treatment must prevent asthma triggers and should be multitarget. However, all current treatments are either mono-target, symptomatic, chemical, and/or instant-relief bronchodilators, such as Salbutamol. We aimed to conceive an osmotically active, stable, non-irritant, and safe nasal surface film to simultaneously protect, clean, and remove inflammatory cytokines, reduce nasal mucosa inflammation and reconstitute the natural defensive barrier of the nasal mucosa, to minimize the intensity, frequency, and duration of asthma exacerbations as a preventive measure.
Patients and methods: We rendered a glycerol osmotic solution base filmogen stabilized with specific glycerol binding natural inert polymers. These polymers were further screened to identify inflammatory cytokines with sandwich ELISA. The filmogen solution was filled in 15 ml nasal sprays (Asmidine®, 125µl/spray t.i.d.) and its preventive efficacy and safety were compared to Salbutamol (100 µg/dose, 1-2 oral puffs, t.i.d.) for 84-days, in 45 comparators and 43 Asmidine® treated patients, as per the GINA recommendations. The trial was approved by the ethical committee and was registered under n°:CTRI/2021/06/034142 (http://ctri.nic.in) by Mudra Clincare, Mumbai, India.
Results: Both test products markedly increased Peak Expiratory Flow Rate (PEFR) and Forced Expiratory Volume (FEV1), Controlled Bronchial Asthma (BA), improved quality of life of the patients as per the SF-36, reduced the need for SABA and minimized the frequency of asthma exacerbations, without any drug-related adverse effects. The efficacy of Asmidine® was progressive and only slightly lower compared to Salbutamol.
Conclusions: Asmidine®, registered as a new generation of asthma prevention medical device in Europe, is the 1st multitarget preventive treatment against asthma and can be used in association with drugs used to treat asthma.
Keywords
Asthma • Prevention • Adults • Polymers • Multi-Target• Safe • Medical Device
Introduction
The Global Initiative for Asthma 2022 (GINA, 2022), considers asthma as one of the most common and fastest-growing epidemics in the world, affecting on average of 9% of the population in different counties [1]. Child and women are more prone to asthma [2]. High concentrations of airborne particles in congested cities coupled with highly irritant car exhaust ozone-rich “smog”, is considered the main cause of triggering and worsening asthma [3]. Allergens with protease activity (dust mites-Der p 1 & 2) are highly cytotoxic to the epithelial barrier, whereas other common allergens can trigger instant immune inflammation. Allergens such as Amb A1 (ragweed), Bet v1 (birch,), Alt a1 (Alternaria, fungus allergens), Fel d1 (cat dander), Phl p5 (timothy grass), and Blot t5 (storage mite), act identically by triggering immune response and inflammation, through the release of cytokines [4,5].
Classically, the cardinal symptoms of asthma include wheezing, breathlessness, chest tightness, and coughing. According to the GINA guidelines to diagnose asthma, clinicians can perform a variety of tests which start with a detailed history/examination of asthma symptoms, measurement of Forced Expiratory Volume in 1 second (FEV1) using a spirometer, positive Bronchodilator (BD) reversibility test performed 10 minutes-15 minutes after 200 mcg-400 mcg salbutamol intake (or equivalent) compared with pre-BD readings, variability in peak expiratory flow rates, positive exercise, and bronchial challenge tests. Asthma control assessment is done by quantifying the frequency of symptoms, use of short-acting beta-2 agonistsagonist, and activity limitation assessment by using control tools such as the Asthma control test and Asthma Control Questionnaire (ACQ-5) [1].
In both allergy and asthma, the disease-triggering factors primarily encounter the Nasal Mucosa (NM) which is the most fragile, abundantly vascularized, and receptor-rich organ in the body [6,7]. Chronic NM damage and inflammation progressively affect the Lower Respiratory System (LRS) with bronchial hyperresponsiveness, contraction of respiratory smooth muscle cells, and reversible airflow obstruction. The symptomatic differences between allergy and asthma may lie in the degree of non-specific hyperresponsiveness of bronchial mucosa, which is nearly 50 times higher than normal in asthma compared to only 2 times-8 times in allergic rhinitis. This may be due to the release of high concentrations of disease-specific key inflammatory mediators such as histamine in allergy and Thymic Stromal Lymphopoietin (TSLP) in asthma [8]. TSLP is now considered a key asthma cytokine as it triggers the release of other proinflammatory interleukins (ILs) such as IL-25 and IL-33 which activate dendritic cells, including differentiation of naïve T cells to Th2 cells, with the abundant release of IL-4, IL-5, and IL-13.9,10 This leads to severe NM inflammation, rupture of the NM epithelial cell barrier, IgE production by B cells, degranulation of mast cells, airway eosinophilia, and mucus hypersecretion, resulting in airway Smooth Muscle Cell (SMC) contraction and consequently respiratory distress [9,10]. This explains why these last few decades, there has been an explosion of scientific research on the probable use of TSLP inhibitor drugs for the treatment of asthma.
The physiopathology of asthma shows that it’s a chronic multifactorial, immunological disease that can be triggered by various immune stimulating factors entering the body, mainly during respiration. Being a highly complex, chronic, and uncurable disease, there are no preventive or curative treatments, but only acute symptomatic treatments available against asthma. The GINA 2019 treatment guidelines are established only to control asthma using pharmacologic and nonpharmacologic interventions to improve the quality of life and prevent exacerbations in patients with asthma. Common medication options include Inhaled Corticosteroids (ICS), long-acting bronchial SMC relaxants, Short-Acting Beta2 Agonists (SABAs), leukotriene receptor antagonists, and oral corticosteroids [11,12]. In the past two decades, multiple individual anti-cytokine Monoclonal Antibodies (mAb) therapies such as AntiImmunoglobulin (Ig) E, Anti-Interleukin (IL)-4 receptor α subunit, anti-IL-5, anti-IL-5Rα, anti-IL-6, anti-IL-33, and anti-TSLP have been developed for treating severe asthma. All these drugs directed to block one of the cytokines of asthma provide only a modest reduction in asthma exacerbation rates, i.e., approximately 40%–60%, and their long-term side effects are still not evaluated. All these chemical or biological treatments, acting on one of the cellular physiological functions, cannot be safe as they also affect other cellular parameters, even if the pharmaceutical industries claim reasonable safety [13].
Being a disease that severely affects the quality of life, patients just need a device or drug, which is safe, and which can minimize the frequency, intensity, and/or duration of attacks which can help them reduce the use of other toxic chemicals or biological drugs and improve Quality Of Life (QOL).
The best hypothetical approach consists in protecting the NM against asthma-triggering factors; and /or simultaneously reducing the concentration of immune cells, TSLP, and other pro-inflammatory cytokines from the NM surface to minimize NM inflammation and to provide ideal conditions for rapid NM natural repair. This multitarget therapeutic approach should automatically diminish the intensity, frequency, and duration of asthma attacks, and in turn the need for chemical treatments to improve the quality of life of patients. As no single chemical entity can fulfill these basic and multiple requirements, we envisaged conceiving a flexible, resistant, non-irritant, osmotically active, and relatively stable liquid polymeric film, capable of forming a protective barrier over the nasal mucosa surface. Being filmogen, the film should act as a long-duration barrier to minimize contact between asthma-triggering factors and the NM. Similarly, being osmotic, the film should attract hypotonic liquids from the NM surface to drain nasal surface contaminants, including multiple proinflammatory cytokines towards the film, where they can be trapped. Repairing and cleaning the NM continuously should reduce inflammatory cascade, cytokine release, and in consequence, the frequency, intensity, and duration of asthma attacks.
The clinical efficacy and safety of this new generation of the device for asthma prevention compared to the most commonly used drug salbutamol, is described in this paper.
Materials and Methods
The conception conception of an osmotic and stable nasal film
The technology used to conceive the osmotic glycerol-basedglycerol based, contaminanttrappingcontaminant trapping, stable, and nonirritant polymeric film has already been described by Shrivastavaetal [14,15].
Selection of osmotic filmogen ingredients
To find an ingredient that can be used to obtain a film openfilmogen liquid for nasal application which is osmotic, non-cytotoxic, absorbent, and stable for 4 hour-6 hour; multiple natural and synthetic ingredients were tested for osmotic and cytotoxic potential using a multi-cellular live cell membrane dehydration model [10]. None of the ingredients met all the criteria except for glycerol which was osmotic and cell-friendly but was slightly irritant and poorly filmogen. The irritant potential of the solution was minimized by adding small quantities of natural thickening agents without affecting the osmotic potential.
Selection of dual-acting polymers to bind with glycerol and with selected proinflammatory cytokines
Glycerol associated with jellifying agents was an excellent osmotic solution, but the resultant film was not stable due to osmotic flow-generated physical pressure, leading to rapid dilution and disintegration of the film within a few minutes. The film was also not capable of trapping protein molecules (cytokines) entering the film through osmosis. To render the osmotic solution film Rogen stable, a small quantity (<0.60%) of certain specific dual-acting polymeric extracts was incorporated into the osmotic glycerol solution. Natural (eg. plant tannins) and synthetic polymers are known to bind with selected macromolecules and with specific proteins (H, OH binding) [16]. The polymer–glycerol molecule binding was evaluated by mixing dried polymeric or tannin-rich plant extract powders (n=182) into the glycerol solution. Polymers that which had an affinity for glycerol molecules were further screened using sandwich ELISA tests to evaluate their affinity to bind with TSLP, IL-4, Il-13, IL-25, IL-33, and IgE cytokine proteins (6-wells per dilution, n=minimum 3), as described by Shrivastava et al. [7] The right quantity of the selected protein binding polymeric mix was then incorporated in glycerol. When spread on a live biological surface, the polymer-bound, specific protein molecule trapping glycerol, forms a 4 hours-6 hours stable film.
Clinical study design
Type of study performed
An 84 days, open-label, randomized, comparative study to evaluate preventive efficacy and safety of the test product (Asmidine®) nasal spray versus Salbutamol metered-dose inhaler, in patients with partially controlled asthma. The first patient was recruited end of June and the study ended in mid-December 2021.
Clinical trial oversight
The study was sponsored by VITROBIO France and was performed by MUDRA CLINCARE, Koparkhairane, Navi Mumbai-400709, India as per the Global Initiative For Asthma (GINA) committee recommendations for such studies. The protocol was approved by relevant ethics committees (Altezza Institutional Ethics Committee, Shree Ashirwad Hospital, Dombivli, Maharashtra, India) and institutional review boards. The trial was registered under n°: CTRI/2021/06/034142 (http://ctri.nic.in) on the 10th of June 2021. A few children between the ages of 8 years-18 years were also enrolled in the trial but they constitute a separate group of patients. The authors vouch for the conduct of the trial, adherence to the protocol, the accuracy and completeness of the data, and the reporting of adverse events. The trial complied with the International Conference on Harmonization Guidelines for Good Clinical Practice, the principles of the Declaration of Helsinki, and relevant national and local regulations. At the time of screening, participants signed written informed consent. The sponsor provided the trial medication and supplied relevant product investigational information.
Study population
The aim was to assess nearly 100 adult patients including a minimum of 40 patients in the Asmidine® test product group and 40 in the Salbutamol comparator group
Inclusion and exclusion criteria
At the time of recruitment, patients were examined physically, their medical history was checked and vital parameters such as blood pressure, heart rate, respiratory rate, and body temperature were measured. The main inclusion criteria were patients aged between 18 years and 70 years; diagnosed with persistent and insufficiently controlled Bronchial Asthma (BA) at least 6 months before the screening visit; having above 2 asthma symptoms weekly with nocturnal awakening; requiring rescue medication more than twice a week, having activity restriction due to bronchial asthma (BA), having to mean Asthma Control Questionnaire-5 (ACQ-5) test index in a range of ≥ 0.75 and <1.5; Forced Expiratory Volume in 1 second (FEV1) before the use of bronchodilators >60% and not under low-dose inhaled Glucocorticosteroids (iGCS) therapy for minimum 2 months before screening. The main exclusion criteria were patients with the need for maintenance therapy of BA; contraindications to iGCS, hypersensitivity to terbutaline, salbutamol, or any components of the study product; diagnosis of Chronic Obstructive Pulmonary Disease (COPD); recording unexpected deterioration of BA symptoms; having pulmonary tuberculosis and heavy smokers.
Randomization
After screening, patients meeting all the inclusion criteria and none of the exclusion criteria were randomized into 2 arms using SAS Version 9.1.3, following a randomization schedule. Block Randomization methodology was employed for generating the list. Within the block, the treatments were distributed in a ratio of 1:1. Each patient received a unique screening identification number, randomization code, enrollment identification number, and a personal diary for daily recordings.
Product Presentation and Application
The test product Asmidine® was supplied by VITROBIO SAS, France (ISO 13485 certified) in 15 ml plastic containers (± 125 sprays; 120 µl/spray) and contained a slightly viscous, brownish liquid. Asmidine® was used by applying 2 sprays in each nostril, t.i.d. for up to 84 days.
albutamol, a short-acting β2 adrenergic receptor agonist bronchodilator, which relaxes airway smooth muscles and is used as a preventive or symptomatic treatment was purchased from commercial sources (ASTHALIN inhaler from Cipla India Ltd., containing salbutamol 100 mcg/dose, 200 metered doses/inhaler) and was used by inhaling 1 or 2 oral puffs, t.i.d. up to day 84 [17].
The choice of regimen and duration of therapy corresponds to the recommendations presented in the GINA 2018 and the Federal Clinical Guidelines for the diagnosis and treatment of BA [18].
Parameters recorded
Patients’ health-related parameters were recorded at randomization visit 1 (week 1), visit 2 (week 4), visit 3 (week 8), and visit 4 (week 12). The key parameters recorded were physical and vital signs (heart rate, blood pressure, breathing rate, body temperature), hematological, blood biochemical, urinalysis, and pregnancy tests (only in adult females), at each visit.
Asthma-related parameters such as assessment of forced expiratory volume in 1 second (FEV1), which measures the maximum amount of air the patient can forcefully exhale in one second, was recorded through spirometry, and Peak Expiratory Flow Rate (PEFR1), indicating the maximum air flow achieved during a forced expiration starting from the level of maximal lung inflation, was measured with a peak flow meter at each visit. In addition to adverse event recording, the BA control assessment was done according to ACQ-5, and quality of life parameters was assessed with the SF-36 eight-scale questionnaire based on Physical Functioning (PF), Role-Physical (RP), Bodily Pain (BP), General Health (GH), Vitality (VT), Social Functioning (SF), Role-Emotional (RE), and Mental Health (MH)), on a 0-100 scale. Mean values were calculated; lower scores indicated higher disability.
Study endpoints
The preventive efficacy of Asmidine® compared to Salbutamol was evaluated at visits 1, 2, 3, and 4 by comparing the change in mean PEFR and FEV values (including 2h after 1st product administration on the 1st and last day for FEV), average weekly need for SABA (terbutaline preparation); and the number of patients with exacerbations at visits 2, 3, and 4. The changes in the ACQ-5 index and Quality-of-Life SF-36 questionnaire at the end of the study were compared with starting baseline values. The number of patients reaching BA control (<0.75 index according to the ACQ-5 questionnaire) was measured at Visit 4.
Safety Endpoints included the total number of AEs (Adverse Events) by severity and frequency, the occurrence of AEs and SAEs (Severe Adverse Events) associated with the use of the study/ reference product, the number of patients with at least one registered AE, and the proportion of patients who discontinued treatment due to AE in each group.
Statistical analysis
Statistical analyses for most pharmacological experiments were performed in GraphPad Prism 8.2.1 (GraphPad Software, Inc., CA, USA). Cell survival in glycerol film and cytokine binding were analyzed by one-way ANOVA followed by the post hoc Dunnett’s multiple comparison test. Inhibition of luminescence due to the binding of polymers or excipients with disease-specific proteins was analyzed by one-way ANOVA followed by the post hoc Bonferroni test. A pvalue <0.05 was considered statistically significant, with a Confidence Interval (CI) of 95%.
For clinical parameters, a change in FEV1, the volume of air exhaled during the first second of forced exhalation between visits 4 and 1 was used as a primary efficacy endpoint. Comparison of the parameters in both groups of patients was performed by calculating a 95% confidence interval for the difference of μe and μs, where μe and μs are the mean values of FEV1 change compared to the baseline values in the groups of patients receiving study and reference products, respectively (μ corresponds to the difference between FEV1 at visits 4 and 1). The study product is considered non-inferior to the reference product if the lower limit of 95% confidence interval for the difference of and is greater than ml. as per FDA guidelines for choosing a margin of non-inferiority in asthma clinical studies.
The statistical analyses were based on the null hypothesis (Hº) where treatment with the use of the study product is inferior or the HA hypothesis where it is superior to the treatment with the reference product. The sample size for such a comparative study was calculated by a statistician.
Results
Selection of specific cytokine binding polymers and conception of asthma prevention osmotic film
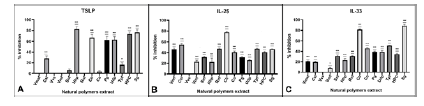
Among 182 natural tannin-rich plant extracts and synthetic polymers, only 32 were able to bind with glycerol and render the glycerol solution film open at low (<1.0%), non-cytotoxic concentrations. Among the glycerol-binding plant polymer extracts, Vvs, Clr, UDP, and Tpf were able to bind with one or more asthma disease-specific proteins, mainly TSLP, IL-25, IL-33 as well as IL-4, IL-13, and IgE (Figure 1). The minimum active concentration of active polymers was then incorporated in the filmogen glycerol to prepare a sufficiently liquid sprayable solution [15].
The aim was to conceive a sprayable film-forming solution, containing minimum concentrations of a polymeric mix (<0.60%) and capable of trapping a maximum number of asthma-specific proteins when these protein molecules enter the film. As shown in Figure 1, TSLP was preferentially trapped by Clr (65.3 ± 6.3%) and Udp (61.8 ± 5.2%); IL-4 by Vvs (85.4 ± 4.7%) and HPC (95.0 ± 8.1%); IL-13 by Clr (92.5 ± 8.4%) and Sg (61.8 ± 3.8%); IgE by Clr (88.2 ± 7.6%), Udp (81.1 ± 5.5%) and Tpf (59.3 ± 4.9%); and IL-33 by Sg (85.9 ± 6.6%) and Clr (78.0 ± 6.3%). A few polymers showed no binding with any proteins, indicating that polymers are highly specific concerning their affinity for proteins.
Figure 1: Key asthma disease specific proinflammatory cytokines
Taking into consideration the neutralization of disease proteins and the maximum usable concentrations for each polymer, 0.60% of protein binding polymeric mix was incorporated in a 7.7% glycerol base in water to conceive an Asmidine® solution. The asthma preventive properties of Asmidine® vs Salbutamol were then evaluated in partially controlled asthma patients.
The key asthma disease specific pro-inflammatory cytokines (ex; TSLP, IL-25, IL-33) were incubated for 5 min with each at a concentration of 0.10% and % inhibition of cytokine activity was evaluated with ELISA sandwich assay (n=18 per test). Results were statistically analyzed using one-way ANOVA followed by Dunnetts post hoc tests. Confidence intervals 95%. *p<0.05 **p<0.01 p<0.001.
Clinical trial result
Demographics
The trial demographic distribution is shown in theconsort diagram. Among 99 patients enrolled for screening, 10 patients failed the screening test and 89 were randomized, 45 in Salbutamol and 44 in the Asmidine® group. 2 patients in the Asmidine® group moved to another region and discontinued the intervention. The final analyses at week 12 were performed on 45 patients (30M+15F, mean age 39.3 ± 13.91 yrs) in Salbutamol and 42 (20M+22F, mean age 40.3 ± 11.82 yrs) in the Asmidine® group. There were fewer males in Asmidine® vs Salbutamol group and this difference was taken into consideration while interpreting respiratory parameters.
Effect on Peak Expiratory Flow Rate (PEFR)
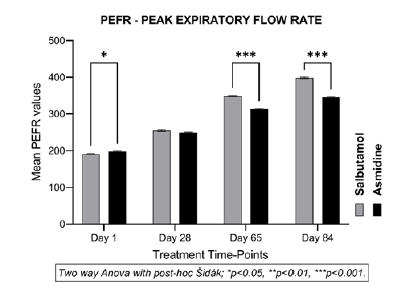
The PEFR is an indication of the capacity of the lungs to accommodate air which is drastically reduced in asthmatic patients. The normal peak flow is 450 L/min-550 L /min in adult males, and 320 L/min-470 L/min in adult females [18]. Accordingly, the mean peak flow in adults is considered between 385 L/min-510 L/min, with an average of 447 L/min. The baseline week-1 mean PEFR values at the start of treatment were 198.0 (± 10.80) in the Asmidine® group vs 191.4 (± 9.08) in the Salbutamol group (Figure 2). These scores were low but met the recruitment criteria. A slow and progressive statistically significant (p<0.001) increase in mean PEFR values vs baseline was noticed at week 4 in Asmidine® (250.0 ± 9.04) and Salbutamol (255.3 ± 15.79) groups indicating beneficial effects of both treatments within 4- weeks. The difference between the two groups was not significant.
Compared to baseline values, the mean PEFR after 8, and 12 weeks of treatment in the Asmidine® group went up to 313.2 (± 10.5) and 345.5 (±11.36) showing an increase of about 25% and 38% compared to baseline. In the Salbutamol group, the mean PEFR was 348.9 (± 12.52) and 398.2 (± 13.28) at weeks 8 and 12 indicating an improvement of about 82% and 108% vs baseline values showing normalization of PEFR after 12 weeks of treatment. Although the PEFR increase in both groups after 8 and 12 weeks was statistically significant vs baseline (p<0.00), the increase with salbutamol vs Asmidine® was significant (p<0.001) at weeks 8 and 12. It should be noted that PEFR in males is higher compared to females and there were 50% more males in the salbutamol-treated group. Being a short-acting beta (2)-agonist, Salbutamol exerts a quick broncho-dilating effect but continuously inhaling a low dose of the drug can exert preventive effects. The efficacy of Asmidine® is slightly lower vs Salbutamol but being a mechanically acting, non-chemical drug, the results with Asmidine® are highly encouraging.
The Peak Expiratory Flow Rate (PEFR/PEF1), indicating the maximum air flow rate generated during a forceful exhalation, starting from full lung inflation, was measured with a peak flow meter from baseline to day 84. The mean PEFR1 in total population with statistical difference (two-way Anova/Sidak; *p<0.05, ** p<0.01, and *** p<0.001) between the Salbutamol and Asmidine® groups at each endpoint compared to mean baseline value (Figure 2).
Forced Expiratory Volume (FEV1)
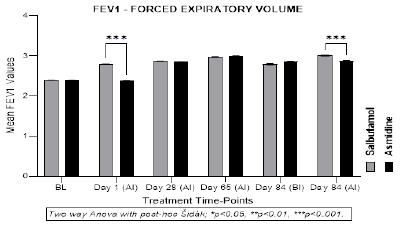
FEV1 is the amount of air forced from the lungs by an individual in one second which is reduced in case of air-flow obstruction of the respiratory tract in asthma patients. Normal values of FEV1 in healthy M aged 20-60 range between 3.5 L to 4.5 L (mean 4 L) and 2.5 L to 3.25 L (mean 2.87) in F, with an average FEV1 value of 3.4 L per second [19,20]. The mean FEV1 was 2.40L in both groups at baseline.
2h after the 1st and last treatments, Salbutamol patients showed a statistically significant mean increase in FEV1 (2.80 ± 0.02; p<0.001 vs baselines), but Asmidine® had no effect which may be related to the fact that Asmidine® is a preventive but not instantly acting bronchodilator (Figure 3).
After 4, 8 and 12 weeks of continuous treatment, a statistically significant progressive increase in FEV1 was seen in both groups up to week 12 (visit 4). The mean increase was 19%, 25%, and 19% in Asmidine® vs 19%, 24%, and 17% in Salbutamol, after 4, 8, and 12 weeks, respectively. FEV1 measurements taken in both groups 2h after the last product administration showed no change compared to values -2 h before treatment in the Asmidine® group but a slight increase (3.01 vs 2.80L; +7.5%) was observed in Salbutamol treated group. These results show that the efficacy of both treatments in progressively improving FEV1 is comparable, but the onset of effects is much quicker in the Salbutamol group. These results, coupled with PEFR (capacity of lungs to accommodate air), prove that both Salbutamol and Asmidine® remarkably improve lung respiratory parameters in asthma patients but Asmidine® cannot be used as an instant relief treatment during an asthma crisis.
The maximum amount of air expelled from the lungs within 1 second (FEV1) was measured with a spirometer at each visit. Mean FEV1 in and total population with statistical differences (two-way Anova/Sidak; *p<0.05, ** p<0.01, and *** p<0.001) between the Salbutamol and Asmidine® groups at each visit compared to mean baseline value (Figure 3)
Asthma Control Questionnaire (ACQ-5)
As per GINA recommendations, lung functions were scored employing a grouped questionnaire, representing 5 items, where a score <0.75 indicates well-controlled asthma, and >1.5, poorly controlled asthma [21]
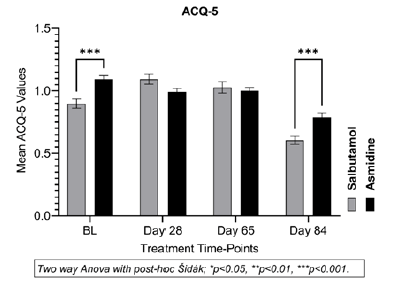
At the start of the study, the mean ACQ-5 score of the Asmidine® group was 1.1 ± 0.18 compared to 0.90 ± 0.25 (p<0.001) in the reference Salbutamol group. There were no significant changes in mean ACQ scores up to the end of week 8 in both groups (values between 1.0 to 1.1) but at week 12, the mean ACQ-5 scores were 0.80 (± 0.20) in Asmidine® and 0.60 (± 0.22) in Salbutamol groups indicating that controlling bronchial asthma in both groups takes time (Figure 4).
Figure 4: Asthma Control Questionnaire (ACQ)
The mean scores indicate, well controlled (ACQ ≤ 0.75), partly controlled (0.75>ACQ ≤ 1.5) or uncontrolled (ACQ>1.5) asthma.Statistical difference (two-way Anova/Sidak; *p<0.05, ** p<0.01, and *** p<0.001) between the Salbutamol and Asmidine® groups at each endpoint compared to mean baseline value (Figure 4).
The number of patients in each group having BA controlled at the start and the end of the study are shown below (Table 1).
Table 1: BA control ACQ-5 score range and the number of patients in each group at weeks 4 and 12
| ACQ-5 mean score | N° patients Asmidine | N° patients Salbutamol | ||
|---|---|---|---|---|
| Baseline (n=44) |
Week 12 (n=42) |
Baseline (n=45) |
Week 12 (n=45) |
|
| Not controlled >1.5 | 0 | 0 | 1 | 0 |
| Partially controlled >0.75<1.5 | 43 | 30 | 37 | 18 |
| Well-controlled <0.75 | 1 | 12 | 7 | 27 |
At the start of the study, 1/44 patients in Asmidine® and 7/44 in the Salbutamol group had well-controlled BA but at the end of week 12, 12/42 patients in Asmidine® and 27/42 in Salbutamol groups had ACQ-5 scores <0.75, indicating well-controlled BA while other patients had partially controlled BA (Table 1). These results show that both treatments help achieve controlled BA but the effects are slow and progressive and Salbutamol is slightly more efficient v:s Asmidine® in controlling BA.
Quality Of Life (QOL) assessment via SF-36 (Short Form Survey)
SF-36 questionnaire (Short Form Survey-36) is a non-specific questionnaire for the assessment of overall well-being and degree of satisfaction with the aspects of human activity in which 36 questions are grouped in 8 QOL parameters [22]. As shown in Table 2, both Asmidine® and Salbutamol treatment for 12 consecutive weeks significantly improved QOL. Compared to baseline, Asmidine® treatment improved physical functioning by 24.4% (p<0.001), role limitations due to physical health by 2.7% (NS), role limitations due to emotional problems by 15.3% (p<0.001), improved energy / less fatigue by 6.4% (p<0.001), emotional wellbeing by 5.0% (p<0.05), social functioning by 11.6% (p<0.01), reduced pain sensation by 12.1% (p<0.001), and overall general health by 25.2% (p<0.001). Salbutamol treatment equally considerably improved QOL, and the improvements are closely comparable to those of Asmidine®. These results, evaluated after 12 weeks of treatment, show that Asmidine® is nearly as good as Salbutamol in improving the QOL of asthmatic patients. This improvement in both groups reflects the concomitant improvements in other respiratory parameters observed during the study.
A two-way ANOVA followed by the Sidak's multiple comparisons test for comparison between the investigational group and comparator group (*p<0.05, **<0.01, ***p<0.001) (Table 2).
Table 2: Parameters are presented as mean ± SD
| Mean values (SF-36 Scales) | Asmidine (n=44) | Salbutamol (n=45) |
|---|---|---|
| Mean value, baseline (day 1) | ||
|
Physical functioning |
49.5 (± 9.69) | 52.8 (± 10.31) |
|
Role limitations due to physical health |
82.4 (± 14.84) | 72.2 (± 23.97) |
|
Role limitations due to emotional problems |
72.0 (± 26.85) | 70.8 (± 22.85) |
|
Energy/fatigue |
51.5 (± 6.43) | 52.1 (±6.08) |
|
Emotional well-being |
50.6 (± 9.25) | 50.9 (± 8.11) |
|
Social functioning |
45.5 (± 11.47) | 46.1 (± 11.56) |
|
Pain |
57.2 (± 7.12) | 57.0 (±4.76) |
|
General health |
42.7 (± 5.55) | 42.1 (± 6.70) |
|
Mean value, (day 84) |
Asmidine (n=42) | Salbutamol (n=45) |
|
Physical functioning |
73.9 (± 14.12) | 71.8 (± 14.15) |
|
Mean change from baseline |
24.4± 4.43 | 19.0 ± 3.84 |
|
Difference vs. comparator |
-2.15 | |
|
Role limitations due to physical health |
85.1 (± 16.62) | 82.8 (± 21.86) |
|
Mean change from baseline |
2.7± 1.78 | 10.6 ± 2.11 |
|
Difference vs. comparator |
-2.34 | |
|
Role limitations due to emotional problems |
87.3 (± 17.95) | 79.3 (± 20.46) |
|
Mean change from baseline |
15.3± 8.9 | 8.5 ± 2.39 |
|
Difference vs. comparator |
-8.03 | |
|
Energy/fatigue |
57.9 (± 9.51) | 58.1 (± 9.19) |
|
Mean change from baseline |
6.4± 3.08 | 6.0 ± 3.11 |
|
Difference vs. comparator |
(-0.25) | |
|
Emotional well-being |
55.6 (± 9.83) | 54.4 (± 8.33) |
|
Mean change from baseline |
5.0 ± 0.58 | 3.5 ± 0.22 |
|
Difference vs. comparator |
-1.22 | |
|
Social functioning |
57.1 (± 15.16) | 63.1 (± 14.83) |
|
Mean change from baseline |
11.6 ± 3.69 | 17.0 ± 3.27 |
|
Difference vs. comparator |
(-5.91) | |
|
Pain |
69.3 (± 16.58) | 68.1 (± 14.2) |
|
Mean change from baseline |
12.1 ± 9.46 | 11.1 ± 9.44 |
|
Difference vs. comparator |
-1.23 | |
|
General health |
67.9 (± 11.95) | 68.8 (± 12.12) |
|
Mean change from baseline |
25.2 ± 6.4 | 26.7 ± 5.42 |
|
Difference vs. comparator |
(-0.92) | |
| Parameters are presented as mean ±SD. A two-way ANOVA followed by the Šídák's multiple comparisons test for comparison between the investigational group and comparator group (*p<0.05, **<0.01, ***p<0.001). | ||
| No significant difference was observed between the two groups; however a small but visible variance can be noticed in the results (MEAN±SD) at the end of the treatment (day 84). | ||
No significant difference was observed between the two groups; however, a small but visible variance can be noticed in the results (MEAN ± SD) at the end of the treatment (day 84).
Average weekly need for SABA (ShortActing Β-Agonists, terbutaline preparation)
Between weeks 3 and 4, two patients in the Asmidine® group and 4 in the Salbutamol comparator group required SABA treatment (terbutaline) for a maximum period of 2 days. Thereafter, only 2 patients in the Asmidine® group and 1 in the Salbutamol group required one 1-day rescue treatment with SABA. No patient in any of the groups required acute treatment from week 8 onwards.
These results clearly show that both treatments are highly effective in minimizing the need for acute treatments after 6 weeks -8 weeks of continuous therapy.
Exacerbations
An exacerbation in COPD is a worsening or “flareup” of respiratory symptoms which equally reflects the efficacy and safety of treatments.
Between week 1 and week 4, four patients showed 6 exacerbations in the Salbutamol group and two patients with 3 exacerbations in the Asmidine® group. The incidence reduced to 1 in Salbutamol and 2 patients in the Asmidine® group between weeks 4-8 and there were no exacerbations thereafter. These results indicate that both treatments are well tolerated and helped in reducing COPD exacerbations after 4 weeks-8 weeks of treatment.
Adverse Events(AE)
In both treatment groups, no Serious Adverse Events (SAEs) were recorded. No patients in the clinical trial discontinued the study due to adverse events, 13 individuals in the Salbutamol group and 16 participants in the Asmidine® group experienced only minor side effects.
Rare cases of mild to a moderate stuffy nose, nausea, sneezing, vomiting, sore or irritated throat, bad taste, dry mouth, and headache were observed once or twice in a few patients during the study but the AE disappeared rapidly. Most patients only experienced these adverse effects for one day or less, whilst one patient in the Asmidine® group and another in the Salbutamol group experienced stuffy nose and sore throat symptoms for two days. As all the AEs were transitory and commonly observed in asthmatic patients, they are not considered related to treatments.
Other parameters
The participants in both groups underwent a battery of medical tests including blood chemical analysis, blood count, vital signs, and urinalysis but no significant change compared to baseline data, or between the groups, was recorded at the end of the study.
Discussion
Modern lifestyle has given humans both luxurious life and diseases. Even though scientists all over the world are trying to find new chemical and biological drugs for asthma, such treatments are often symptomatic, not curative, and may have multiple long-term side effects. Currently, there is no preventive treatment that can stop or at least minimize the frequency, intensity, and/or duration of asthma exacerbations. They are prevented, to some extent, only through regular use of bronchodilators, corticosteroids, or single proteintargeted biologicals during the entire disease period. Even if such treatments are claimed to be relatively safe, their long-term regular use represents a considerable health risk. Multiple chemical and biological drugs were found to be unsafe only several years after they had been on the market. For example, long-term side effects were observed with the weight loss drugs dexfenfluramine (redux) and benfluorex (mediator), selective serotonin uptake blockers for depression, diabetic drug rosiglitazone (Avandia), arthritis medication rofecoxib (Vioxx), celecoxib (Celebrex) and even commonly used drugs like aspirin in certain countries. Recent trends of promoting biologics, which are synthesized or extracted from a biological source such as monoclonal antibodies, receptor, or enzyme mimicking modulators, are highly specific to target a particular physiological or biological function but they also affect other cellular physiological functions and may induce undesired effects. Of the 23 biologicals marketed since 1998 in the USA, 13 already got black box warnings [22]. Moreover, none of these drugs take into consideration the complex multifactorial physiopathology of a disease, such as asthma.
Almost all asthma-triggering factors come into contact with the NM, initiating an inflammatory cascade and events leading to bronchial SMC contraction. These events do not allow sufficient time for NM reconstruction and defense, as most of the asthma patients have >2 asthma exacerbations per month, continuously maintaining the disease process and severely worsening the quality of life of patients. Asthma patients know that asthma involves a multifactorial physiopathology and that exposure to asthma-triggering factors cannot be reduced, but still, they hope that modern research will discover a safe and preventive treatment that can help them live a better life
Asmidine® is a safe, osmotic, and absorbent topical film barrier which can protect the NM against incoming asthma-triggering factors for 4hour-6hour, clean the NM continuously, attract and trap free floating NM surface protein molecules, allow rapid NM cell growth and repair, minimize triggering of the inflammatory cascade, and in consequence the frequency, intensity, and severity of asthma attacks.
Clinical trial results show that Asmidine® is only slightly less effective compared to Salbutamol in improving PEFR, FEV1, and in controlling BA while the efficacy of reducing the need for SABA and corticosteroids, and the improvement in QOL are comparable for the two test products. Salbutamol inhalers are generally used as a lifesaving drug during asthma attacks as they relax airway muscles and open the airways to ease breathing for up to 4h. In many cases, the bronchodilator treatment is associated with corticosteroids, long-acting β-agonists, anticholinergics, and leukotriene receptor antagonists, to prevent the occurrence, intensity, and/or frequency of asthma exacerbations or in lower doses, throughout life as preventive therapy [23,24]. These are chemical molecules and cannot be free of undesired effects.
Recently, several new medications, known collectively as “biologics,” have been approved for the treatment of moderate-to-severe asthma. Biologics are unique in that they target a specific antibody, molecule, or cell involved in asthma. Because of this, they are also known as “precision” or “personalized” therapy [25]. The biologics block one or at most, two asthma proteins but not all the cytokines involved in the disease. The key FDAapproved biologics, company, and their cytokine targets are Cinqair (GSK-Teva, reslizumab, IL-5), Dupixent (dupilumab, IL-4, 13), Fasenra (AstraZeneca, benralizumab, IL-5), Nucala (GSK, mepolizumab, IgG1 kappa anti-IL-5), Tezspire (Amgen-AstraZenica, Tezepelumab, TSLP), and Xolair (Novartis, omalizumab, anti-IgE) [24,25]. None of these biologics are preventive or multitarget as they do not protect, clean, or repair the NM surface and block all the asthma chain-reaction generated cytokines simultaneously. The new biological drugs are highly expensive and should be used under strict medical supervision. Daily use of Salbutamol as a preventive therapy is effective, but we should not forget that these drugs are chemicals, that they are administered daily over years, nasal and upper respiratory tract mucosa of asthmatic patients is chronically inflamed, the chemicals are absorbed in the body, and their long-term use sideeffects are not elucidated. The basic question remains: Is long-term daily exposure to chemical drugs safe? In the absence of any alternative, these questions were not raised but the results of polymeric osmotic filmogen technology presented in this study prove that protecting and cleaning the NM as well as continuously removing inflammatory nasal surface proteins from asthmatic NM, provide an excellent means of preventing and controlling asthma. This technology has already been successfully employed for the treatment of nasal, oral, and skin diseases such as influenza, oral mucositis, bedsore & chronic wounds, rhinosinusitis, cough, allergic rhinitis,6 pharyngitis, hemorrhoids, and even Covid-19 [26-32].
Conclusion
Asmidine is a new generation of non-chemical, mechanically acting, and safe nasal spray devices for the preventive treatment of asthma. The product is already registered in Europe as a medical device and should help minimize the lifelong use of chemical drugs for the prevention of asthma.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Author Contribution
All authors made a significant contribution to the work reported, whether that is in the conception,study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
- Global Initiative for Asthma. Global strategyforasthma managementandprevention. (2019).
[Google Scholar] [Crossref]
- Nunes C, Pereira AM, M AM. Asthma costs and social impact. Asthma Res Pract. 3:1(2017). [Google Scholar] [Crossref]
- Enilari O, Sinha S. The Global impact of asthma in adult populations. Ann Glob Health. 85:1(2019). [Google Scholar] [Crossref]
- Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 129(4):1441-51(2019). [Google Scholar] [Crossref]
- Murrison LB, Brandt EB, Myers JB, et al. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest. 129(4):1504-15(2019). [Google Scholar] [Crossref]
- Watts AM, Cripps AW, West NP, et al. Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents. Front Pharmacol. 10:294(2019). [Google Scholar] [Crossref]
- Namazy JA, Schatz M. Asthma, Allergic and Immunologic Diseases During Pregnancy: A Guide to Management. Springer Int Publ. 61-86(2019). [Google Scholar] [Crossref]
- Schaper K, Rossbach K, Köther B, et al. Stimulation of the histamine 4 receptor upregulates thymic stromal lymphopoietin (TSLP) in human and murine keratinocytes. Pharmacological research. 113(Pt A):209-15(2016). [Google Scholar] [Crossref]
- Gauvreau GM, Sehmi R, Ambrose CS, et al. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma.Expert Opin Ther Targets.24(8):777-92(2020). [Google Scholar] [Crossref]
- Menzies-Gow A, Wechsler ME, Brightling CE. Unmet need in severe, uncontrolled asthma: can anti-TSLP therapy with tezepelumab provide a valuable new treatment option? Respir Res. 21(1):268(2020). [Google Scholar] [Crossref]
- Salter B, Lacy P, Mukherjee M. Biologics in asthma: A molecular perspective to precision medicine. Front Pharmacol.12(2022). [Google Scholar] [Crossref]
- Shrivastava L, Schütte H, Malik P, et al. A new class of polymeric anti-allergen nasal barrier film solution for the treatment of allergic rhinitis. J Allergy Ther. 8(3):1-9(2017). [Google Scholar] [Crossref]
- Shrivastava R, Shrivastava R, Johansen B, et al. Anti-inflammatory and antiviral osmotic polymeric film to treat Covid-19 early-stage infection. J Inflamm Res. 14:1195-206(2021). [Google Scholar] [Crossref]
- Shrivastava L, Shrivastava R, Shrivastava R, et al. Dual acting polymers in an osmotic film for topical application to treat inflammatory diseases and cytokine release syndrome. 2022. [Google Scholar] [Crossref]
- Chan AHY, Watkins K, Schneider CR. Management of Respiratory Disorders and the Pharmacist's Role: Asthma. In: Babar Z-U-D, ed. Encycl Pharm Pract Clin Pharm. 244-263(2019). [Google Scholar] [Crossref]
- Global Initiative for Asthma.Global Strategy for Asthma Management and Prevention.2018. [Google Scholar] [Crossref]
- R S, K NP, N K. The peak Expiratory Flow Rate (PEFR): the effect of stress in a geriatric population of chennai- A pilot study. J clin diagn res.7(2):409-10(2013). [Google Scholar] [Crossref]
- Fang LJ. The Impacts of Pulmonary Rehabilitation Therapy on Patients After Thoracic Surgery (VATSMIPMEP). Chang Gung Memorial Hospital; November 3, 2020. [Google Scholar] [Crossref]
- Lin J, Fu X, Jiang P, et al. INITIAL – An observational study of disease severity in newly diagnosed asthma patients and initial response following 12 weeks’ treatment. Sci Rep. 9(1):1254(2019). [Google Scholar] [Crossref]
- The RAND Corporation. 36-Item Short Form Survey (SF-36) Scoring Instructions. (1993). [Google Scholar] [Crossref]
- Resnik DB. Beyond post-marketing research and MedWatch: Long-term studies of drug risks. Drug des dev ther. 1:1-5(2007). [Google Scholar] [Crossref]
- National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Natl Heart Lung Blood Inst. (2007). [Google Scholar] [Crossref]
- Galeone C, Scelfo C, Bertolini F, et al. Precision medicine in targeted therapies for severe asthma: Is there any place for "Omics" technology? BioMed res int. 2018:4617565(2018). [Google Scholar] [Crossref]
- Morris TS, Autry EB, Kuhn RJ. The role of biologics in the management of asthma in the pediatric patient. J pediatr pharmacol ther. 26(5):427-36(2021). [Google Scholar] [Crossref]
- Shrivastava R. A new therapeutic approach to neutralize throat surface proteases and virus glycoproteins simultaneously for the treatment of influenza virus infection. Int J Virol. 7(2):53-63(2011). [Google Scholar] [Crossref]
- Shrivastava R, Deshmukh S. A new therapeutic approach to treat oral mucositis using specific MMP blockers in an osmotically active solution. J Cancer Res Treat. 1(1):4-11(2013). [Google Scholar] [Crossref]
- Shrivastava R, Cucuat N, Rousse M, et al. A new generation of topical chronic wound treatments containing specific MMP inhibitors. Chronic Wound Care Manag Res. 1:31-40(2014). [Google Scholar] [Crossref]
- Shrivastava R, Tourret E, Schutte H, et al. Clinical efficacy of a new filmogen polymeric glycerol solution for the treatment of rhinosinusitis. 3(2):2-8(2017). [Google Scholar] [Crossref]
- Shrivastava R, Carrois F, Pisak M, et al. Clinical efficacy of novel filmogen, antimicrobial, cleaning, fluidizing cough treatment. J clin trials. 7:1-8(2017). [Google Scholar] [Crossref]
- Rousse M, Schutte H, Guy M, et al. A randomized, double-blind, controlled study to evaluate clinical efficacy and safety of novel filmogen osmotic treatment for pharyngitis. Clin. Investig. 7(3)(2017). [Google Scholar] [Crossref]
- Shrivastava L, Borges G, Shrivastava R. Clinical efficacy of a dual action, topical anti-edematous and antiinflammatory device for the treatment of external hemorrhoids. Clin Exp Pharmacol. 8(1):1-7(2018). [Google Scholar] [Crossref]
- Srivastava R, Vijay M, Maneby N, et al. Clinical efficacy of an osmotic, antiviral and anti-Inflammatory polymeric nasal film to treat Covid-19 early-phase respiratory symptoms. Open Access J Clin Trials. (13):11-20(2021).