Review Article - Interventional Cardiology (2021)
Cholesterol crystal induced inflammation and mechanical cardiac valve injury: Implications for transcatheter aortic-valve replacement
- Corresponding Author:
- George S. Abela
Division of Cardiology,
Department of Medicine,
College of Human Medicine,
USA
E-mail: abela@msu.edu
Received date: March 22, 2021 Accepted date: April 05, 2021 Published date: April 12, 2021
Abstract
The process of cardiac valve sclerosis and stenosis in non-rheumatic disease is related to inflammation via the innate immune system similar to atherosclerosis. Cardiac valve composition shares many features with arterial tissue. Cholesterol infiltration in the valve matrix results in cholesterol crystal formation and deposition that causes mechanical injury and triggers inflammation. Sclerotic human valve specimens as well as valves from atherosclerotic rabbit models reveal presence of cholesterol crystals and macrophage infiltration as seen in atherosclerosis. Lipid lowering by combination of simvastatin and ezetimibe demonstrates cholesterol reduction in valves only if used in a preventive manner. However, once the valve is infiltrated by cholesterol and crystals form in the tissue matrix, these become very difficult to extract and lipid lowering is no longer very effective. Furthermore, cholesterol crystals serve as a nidus for calcium phosphate deposition that distorts and stiffens the valve tissue leading to valve dysfunction. During Transcatheter Aortic Valve Replacement (TAVR) procedures, crystals can complicate the procedure by release of crystalline particles causing ischemic cerebral events and rupturing balloons.
Keywords
Cholesterol crystals • Cardiac valves • TAVR
Introduction
The role of inflammation in cardiac valve disease has been long recognized in rheumatic fever causing fusion of valve tissue with eventual sclerosis and calcification leading to valve stenosis and/or regurgitation. The process is triggered by an autoimmune response to an infectious agent usually group A Streptococcus pyogenes [1]. However, the activation of the innate immune system due to non-infectious agents has been recently recognized in the arterial system. Specifically, the activation of NLRP3 by cholesterol crystals has been found to lead to IL-1β production resulting in an inflammatory cascade that leads to the activation of IL-6 and subsequent production of C-reactive protein (CRP) by the liver [2].
Cholesterol crystals form in tissues by an increasing saturation of free cholesterol and changes in the local chemical milieu including a rising pH and a drop in ambient temperature within the physiologic range [3,4]. Specifically, a combination of increasing cholesterol saturation with a low temperature was found to be synergistic in triggering the formation of cholesterol crystals. The phase transformation of cholesterol from a liquid to a solid crystalline state leads to a rapid volume expansion that has the capability of perforating fibrous tissues. Moreover, the sharp tip geometry of cholesterol crystals adds to the potential for mechanical injury. Cholesterol crystals have been found perforating the fibrous caps in human coronary atherosclerotic plaques that had ruptured suggesting a causal process in patients who died from acute cardiovascular events. These findings were not present in patients who had coronary atherosclerotic plaques but had died of other causes [5]. The ability to visualize the effect of cholesterol crystals and their extent in atherosclerotic plaque rupture was made possible by excluding ethanol from the tissue processing protocol for scanning electron microscopy [6].
Because cardiac valves and vascular tissues share similar morphology including an endothelial lining and sub-intimal collagenous tissue, we proceeded to examine if a similar process of mechanical trauma and inflammation associated with cholesterol crystals may also exist for cardiac valves [7].
Crystals in Valve Tissue
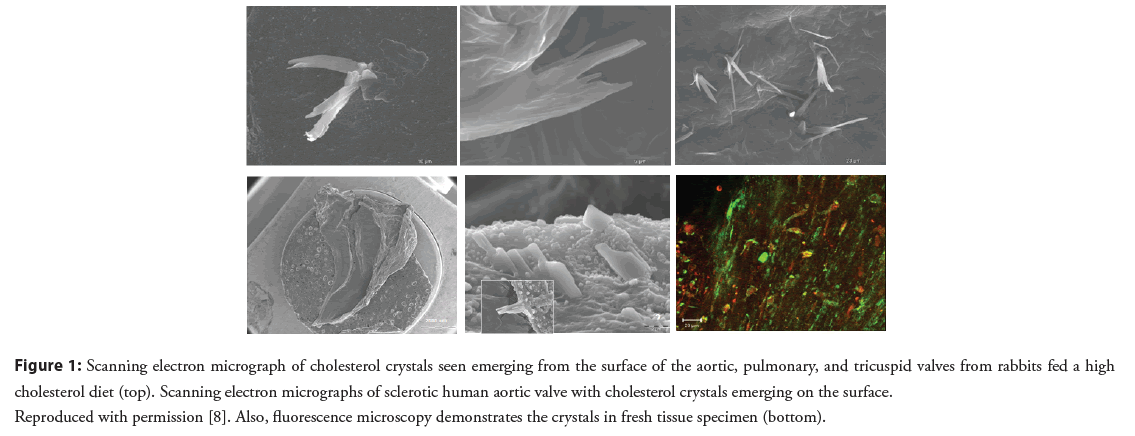
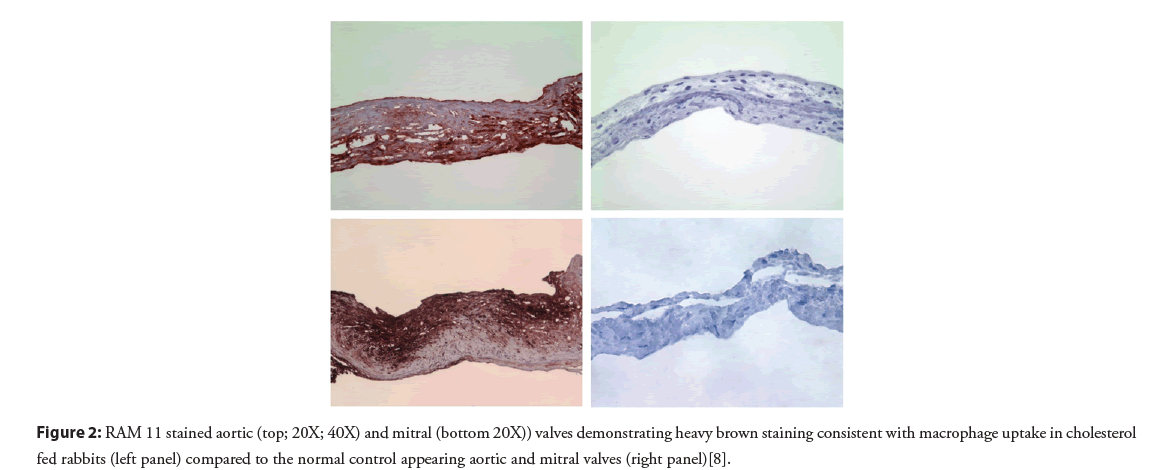
Although the presence of cholesterol crystals in valve tissue obtained at surgery had been previously described, their extent, role in inflammation and valve injury had not been previously recognized. Sclerotic human valves and valves from normal and atherosclerotic rabbits were examined for cholesterol crystals [8]. This demonstrated that cholesterol crystals were present in both human and atherosclerotic rabbit valves (Figure 1). Rabbits fed a cholesterol enriched diet resulted in elevated cholesterol valve content, tissue inflammation and an associated systemic serum inflammatory response. Moreover, valves from the cholesterol diet fed rabbits had extensive cholesterol deposition and macrophage accumulation within the valve tissue matrix as previously described in arterial tissue of atherosclerotic rabbits (Table 1 and Figure 2). Systemically, CRP was markedly elevated in the atherosclerotic rabbits compared to the normal controls. Furthermore, other studies have demonstrated T lymphocytes infiltrating human stenotic aortic valves [9]. This included both bicuspid and tricuspid aortic valves further supporting the inflammatory process within the valve tissue similar to atherosclerosis.
| Treatment group | Aortic (% area) | Mitral (% area) | Pulmonary (% area) | Tricuspid (% area) |
|---|---|---|---|---|
| Atherosclerotic (n=5) | 29 | 35.8 | 28.1 | 7.3 |
| Normal Control (n=12) | 0.1 | 0 | 0 | 0 |
| p-value | 0.002 | 0.02 | 0.002 | 0.002 |
Table 1: Cardiac valve inflammation by percent area staining of macrophage content. Modified with permission [8].
Figure 1: Scanning electron micrograph of cholesterol crystals seen emerging from the surface of the aortic, pulmonary, and tricuspid valves from rabbits fed a high cholesterol diet (top). Scanning electron micrographs of sclerotic human aortic valve with cholesterol crystals emerging on the surface. Reproduced with permission [8]. Also, fluorescence microscopy demonstrates the crystals in fresh tissue specimen (bottom).
Figure 2: RAM 11 stained aortic (top; 20X; 40X) and mitral (bottom 20X)) valves demonstrating heavy brown staining consistent with macrophage uptake in cholesterol fed rabbits (left panel) compared to the normal control appearing aortic and mitral valves (right panel)[8].
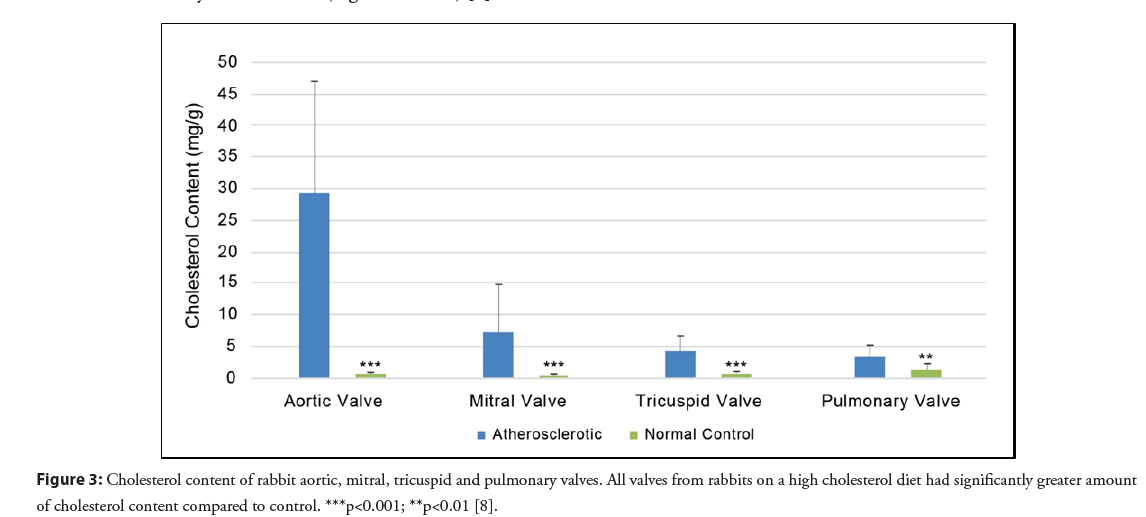
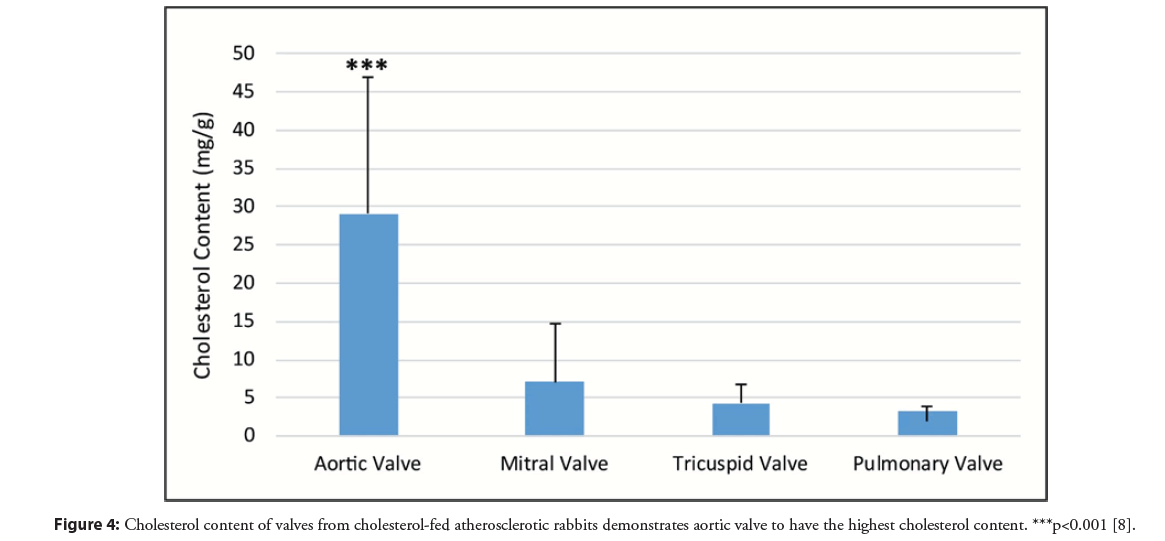
Although all cardiac valves in the atherosclerotic model had a significantly greater amount of cholesterol compared to normal rabbit valves, the aortic valve had the greatest amount of cholesterol accumulation and crystal formation (Figures 3 and 4) [8]. This was followed by the mitral, pulmonic, and tricuspid valves respectively. The aortic valve is typically the most commonly involved valve in humans without rheumatic disease and this may be related to exposure to high pressure at the aortic valve. Additionally, the presence of cholesterol crystals can serve as a nidus for the deposition of calcium phosphate crystals that can further constrict and stiffen the valve restricting its mobility [10]. Moreover, aortic stenosis is associated with an independent higher risk of stroke that is shown to be reduced after valve replacement [11]. Thus, the greater cholesterol and crystal burden in the aortic valve may be a contributor to those cerebrovascular events.
Figure 4: Cholesterol content of valves from cholesterol-fed atherosclerotic rabbits demonstrates aortic valve to have the highest cholesterol content. ***p<0.001 [8].
Lipid Lowering by Simvastatin and Ezetimibe
Although early retrospective studies with statins demonstrated a reduction in the progression of aortic stenosis, prospective studies including a combination of Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) human trial as well as Rosuvastatin in the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) trial failed to slow or prevent the progression of aortic stenosis [12,13]. However, the trials did show benefits in reduced acute coronary events.
In our study, the use of combined simvastatin and ezetimibe in the atherosclerotic rabbits was successful in reducing cholesterol content in the valves and this was associated with reduced valve inflammation and serum CRP [8]. Valve macrophage cell infiltration and CRP were reduced respectively but the beneficial effects were found present only when the simvastatin and ezetimibe were initiated at the same time as the cholesterol enriched diet. However, when treatment was started after establishment of atherosclerosis, the benefit of reduced macrophage infiltration was no longer present.
Once having formed, cholesterol crystals are relatively inert and resistant to being dissolved. This is due to the hydrogen bond energies that hold the crystal molecules together. Only High Density Lipoprotein (HDL) is known to dissolve cholesterol crystals [14]. Once crystals are formed and especially if they are located deep in the valve matrix, their exposure to HDL is limited and they continue to act as a persistent source of inflammation and mechanical injury. We have reported this process of persistent injury in the arterial system with crystals that remain embedded in the tissue as a potential cause for residual risk for cardiovascular events [15].
Potential Influence of Crystals on TAVR
Transcatheter Aortic Valve Replacement (TAVR) has become a well-established approach for replacing aortic valves and the indication has continued to broaden from the original elevated surgical risk patients. However, one important complication of TAVR is cerebral vascular accidents from showering of particulates mainly from the valve during balloon valvuloplasty or valve deployment. Often these events may not be recognized by the patient or physician, but MRI studies have frequently detected subclinical ischemic events from showers of small particulate materials [16-18]. More recent studies have demonstrated persistent embolic events (5%-6%) with TAVR [19,20] and filter devices have been developed to capture debris to reduce embolic events [21]. Moreover, sharp tipped crystals in the valve have the potential of perforating the balloon.
Similar findings of embolic events were described by Hollenhorst who found cholesterol crystal emboli showering from carotid arteries to the ophthalmic circulation to cause amaurosis fugax as well as transient ischemic attacks [22]. We had previously demonstrated that statins and aspirin dissolve cholesterol crystals [23,24] which raises the possibility for intensively premedicating patients undergoing TAVR with statins as well as aspirin prior to the procedure in order to reduce the risk of strokes. Other approaches to reduce lipids and potentially risk of events are PCSK9 inhibitors, bempedoic acid and nutraceuticals diets [25-27].
Discussion and Conclusion
Studies have debated the mechanism for acquired non-rheumatic valvular stenosis and/or regurgitation, specifically for the aortic and mitral valves. However, recently we elucidated the mechanism for these effects by demonstrating that as cholesterol infiltrates the valve matrix forming crystals, they trigger an inflammatory response as noted in the process of atherosclerosis. This occurs by activation of the innate immune system that results in the infiltration of the cardiac valves with macrophages. Also the formation of cholesterol crystals deep in the valve matrix results in a mechanical trauma and calcification causing distortion of the valve structure with stiffening. This process may ultimately lead to valve stenosis and/or regurgitation often requiring valve replacement. Currently, much of the aortic valve replacement is being performed percutaneously via TAVR. Although technically highly successful, micro-emboli to the brain are frequent and remain a significant complication. Intensive lowering of lipids and adding aspirin prior to the procedure may be an approach to consider in reducing cerebrovascular events.
Conflict of Interest
The authors declare that there is no conflict of interest.
Sources of Funding
Support was provided in part from Michigan State University CTSI, The Jean P. Schultz Biomedical Research Endowment and Edward W. Sparrow Hospital, Lansing, MI.
References
- Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2: 15084 (2016).
- Düewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464: 1357-1361 (2010).
- Vedre A, Pathak DR, Crimp M, et al. Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis. 203(1):89-96 (2009).
- Janoudi A, Shamoun FE, Kalavakunta JK, et al. Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque. Eur Heart J. 37(25):1959-1967 (2016).
- Abela GS, Aziz K, Vedre A, et al. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol. 103(7): 959–968 (2009).
- Nasiri M, Janoudi A, Vanderberg A, et al. Role of cholesterol crystals in atherosclerosis is unmasked by altering tissue preparation methods. Microsc Res Tech. 78(11): 969–974 (2015).
- Filip DA, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 59(3): 310-20 (1986).
- Elkhatib L, De Feijter-Rupp H, Janoudi A, et al. Cholesterol induced heart valve inflammation and injury: Efficacy of cholesterol lowering treatment. Open Heart 7: e001274 (2020).
- Wallby L, Janerot-Sjöberg B, Steffensen T, et al. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 88(4): 348-351 (2002).
- Laird DF, Mucalo MR, Yokogawa Y. Growth of calcium hydroxyapatite (Ca-HAp) on cholesterol and cholestanol crystals from a simulated body fluid: a possible insight into the pathological calcifications associated with atherosclerosis. J Colloid Interface Sci. 295(15): 348–363 (2006).
- Greve AM, Dalsgaard M, Bang CN, et al. Stroke in patients with aortic stenosis: The simvastatin and ezetimibe in aortic stenosis study. Stroke. 45(7):1939-1946 (2014).
- Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 359(13): 1343-1356 (2008).
- Chan KL, Teo K, Dumesnil JG, et al. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 121(2): 306-314 (2010).
- Abdulla YH, Adams CW. The action of human high density lipoprotein on cholesterol crystals. Part 2. Biochemical observations. Atherosclerosis. 31(4): 473-480 (1978).
- Nidorf SM, Fiolet A, Abela GS. Viewing atherosclerosis through a crystal lens: How the evolving structure of cholesterol crystals in atherosclerotic plaque alters its stability. J Clin Lipidol. 14(5): 619-630 (2020).
- Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: A diffusion-weighted magnetic resonance imaging study. Circulation. 121(7): 870-878 (2010).
- Armijo G, Nombela-Franco L, Tirado-Conte G. Cerebrovascular events after transcatheter aortic valve implantation. Front Cardiovasc Med. 5: 104 (2018).
- Davlouros PA, Mplani VC, Koniari I, et al. Transcatheter aortic valve replacement and stroke: A comprehensive review. J Geriatr Cardiol. 15(1): 95-104 (2018).
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374: 1609-1620 (2016).
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 376: 1321-133 (2017).
- Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 69(4): 367-377 (2017).
- Bunt TJ. The clinical significance of the asymptomatic Hollenhorst plaque. J Vasc Surg. 4(6): 559–562 (1986).
- Abela GS, Vedre A, Janoudi A, et al. Effect of statins on cholesterol crystallization and atherosclerotic plaque stabilization. Am J Cardiol. 107(12):1710-1717 (2011).
- Vedre A, Aziz K, Huang R, et al. Aspirin Prevents Cholesterol Crystallization: A Potential Mechanism of Plaque Stabilization. J Am Coll Cardiol. 51(SA): 318 (2008).
- Sabatine MS, Giugliano RP, Keech AC, et al. for the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Eng J Med. 376: 1713-1722 (2017).
- Ray KK, Bays HE, Catapano AL, et al. CLEAR Harmony Trial. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 380: 1022-1032 (2019).
- Scicchitano P, Cameli M, Maiello M, et al. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods. 6: 11-32 (2014).