Research Article - Clinical Practice (2021) Volume 18, Issue 4
Role of manual Immature to Total Neutrophil (I/T) ratio and automated Immature Granulocyte Count (IGC) and percentage (ig%) in the early diagnosis of neonatal sepsis
- Corresponding Author:
- Chandima Kulathilake
Department of Pathology
Faculty of Medical Sciences
University of Sri Jayewardenepura, Sri Lanka
E-mail: kulathilake@sjp.ac.lk
Abstract
Background: The diagnosis of neonatal sepsis is challenging due to non-specific and subtle clinical features, low sensitivity and delay in routine laboratory tests. Current study was conducted to evaluate the role of manual Immature/Total (I/T) neutrophil ratio and automated Immature Granulocyte Count (IGC) and Immature Granulocyte percentage (IG%) in the diagnosis of neonatal sepsis. Materials and Methods: An analytical cross-sectional study was done during a period of 6 months with a sample of 55 neonates admitted to Colombo South Teaching Hospital, Sri Lanka. A combination of clinical and laboratory parameters including full blood count, C-reactive protein and blood culture were used to identify the neonates with probable sepsis. The population was subcategorized into five (5) groups and manual Immature/Total neutrophil (I/T) ratio, Immature Granulocyte Count (IGC) and Immature Granulocyte percentage (IG %) were done. Results: The sensitivity of manual I/T ratio was 93.8% and Negative Predictive Value (NPV) of 95.2%. The sensitivity for lower cut off values, IGC of 0.03 × 103/µL and IG% of 0.5% was 80% and 73.3% respectively. The NPV for above cut-off values were 25% and 0.5% respectively. The NPV was improved with higher cut-off values with 70.9% for IGC 0.3 and 70.6% for IG 3%, but sensitivity remained low with 40% and 33.3% respectively. Conclusion: Manual I/T ratio remain as a useful diagnostic tool in diagnosing and excluding neonatal sepsis with a very good sensitivity and NPV. However, further studies and well-defined reference intervals are required in automated IGC and IG%.
Keywords
neonatal sepsis, manual I/T ratio, automated immature granulocytes
Key Message
Neonatal sepsis is a diagnostic challenge and combination of high clinical suspicion and laboratory parameters are used but with many draw backs. Manual IT ratio has shown good results in many studies. Studies on automated immature granulocytes in this regard are few and have shown variable results.
This study confirms further the value of I/T ratio and the findings of automated immature granulocytes have shown variable results.
Introduction
Early onset sepsis is an important cause of morbidity and mortality in neonates [1-3]. The diagnosis is challenging due to non-specific and subtle clinical features in neonates [4]. Although the incidence is low (1.4%-3.2% increasing with decreasing gestational age) the severity of the disease and non-specific nature of early symptoms leads to evaluate and treat many more neonates who actually did not have the disease [5]. In routine practice the diagnosis of neonatal sepsis is by high clinical suspicion supported by laboratory parameters which include positive blood culture, elevated C-Reactive Protein (CRP) and high or low WBC count with immature granulocytes in the peripheral blood smear.
As there are so many drawbacks with currently available laboratory investigations, a test which gives quick results with a good sensitivity, high negative predictive value and cost effective is a need. Immature to Total neutrophil ratio (I/T ratio) which is defined as the proportion of the number of immature cells including blasts, promyelocytes, myelocytes and band forms to the number of total neutrophils determined in a 100 cell-manual differential count in a peripheral blood smear has a long clinical tradition in the diagnosis of early neonatal sepsis [1-10]. Several studies have demonstrated that an IT ratio of 0.2> has a very high negative predictive value (some close to 100%) and a good sensitivity rate [1-9].
Automated Immature Granulocyte Count (IGC) and Immature Granulocyte percentage (IG%), are available in modern hematology analyzers and considered as precise and accurate as drawbacks such as inter- and intra-observer variation of microscopic band cell identification in the above manual differential count is obviated.
In Sri Lanka previous studies on this topic were not available, so that our study wanted to find out the utility of these parameters in a cohort of neonates in our country. We evaluated the significance of Immature/Total neutrophil (I/T) ratio and automated Immature Granulocyte Count (IGC) and percentage (IG%) in the early diagnosis of neonatal sepsis.
Materials and Methods
This was an analytical cross-sectional study conducted over a period of six months. The study group included babies <14 days old who were admitted to Special Care Baby Unit (SCBU) and post natal ward of professorial unit of Colombo South Teaching Hospital during the study period. Neonates who were suspected to have sepsis based on peri-natal risk factors which included low birth weight, prematurity, abnormal amniotic fluid, premature rupture of membranes, foetal distress and maternal pyrexia together with clinical features were taken as the study group.
The clinical evaluation based on peri-natal risk factors and clinical features (e.g. lethargy, poor feeding, change in temperature and respiratory distress) for probable sepsis was done by a consultant pediatrician. A combination of clinical and laboratory parameters was used to identify the neonates with presumed sepsis.
In neonates with clinically presumed sepsis, samples were taken for 0-hour blood culture before starting antibiotics under sterile conditions into BD Bactec Peds Plus blood culture bottles. Samples were incubated in the BD BACTEC fluorescent series instrument followed by manual plating for positive BACTEC samples.
The samples for Full Blood Count (FBC) were taken at the same time into microtainer tripotassium EDTA tubes (approximately 0.5 ml of blood by venipuncture) and analyzed in the Mindray BC 6800 analyzer in the manual mode within 4 hours of collection to obtain total WBC count, immature granulocyte Count (IGC) and immature granulocyte% (IG%). Mindray BC 6800 IGC included promyelocytes, myelocytes and metamyelocytes (not band forms). Those immature forms were detected by the machine using three principles; (1) Forward scatter for cell volume, (2) side scatter for granularity and (3) fluorescence with high affinity for nucleic acid, and when excited by a 635 nm laser beam, the cells fluorescence proportional to their content of nucleic acid. IGs show an intense fluorescence from which they can be separated from mature neutrophils. The machine was calibrated and a quality control sample for WBC and IGs was analyzed daily.
Blood smears were made manually, stained with leishman stain and two 100-cell manual differential counts were done using oil immersion object of Olympus CH-30 microscope at a final magnification of 1000. The manual IGC included promyelocytes, myelocytes, metamyelocytes and band forms. Immature/Total neutrophil (I/T) ratio was calculated for each smear.
The samples for CRP were taken at 48 hrs into plain bottles and sent for analysis. As there was no single laboratory parameter available as the diagnostic tool in early neonatal sepsis; high clinical suspicion, positive blood culture and increased CRP value (>6 mg/L) were considered.
The neonates were further categorized into 5 groups after the results of blood culture and CRP as (1) neonates with definitive sepsis with positive blood culture, (2) neonates with probable sepsis with strong clinical history and positive CRP but negative blood culture, (3) neonates who were investigated and treated due to high clinical suspicion, but no positive laboratory parameters, (4) neonates who were investigated but not treated for sepsis and (5) sepsis not suspected and FBC taken for other reasons. We did not include CSF culture as a laboratory parameter in our study as in early neonatal sepsis work up, CSF analysis is done as a second line investigation, only when there is a clinical suspicion of CNS sepsis (e.g. fits or other neurological signs) or if there is positive blood culture. Urine culture was also not included as in newborn babies, uro-sepsis is not common and it is done as a part of septic screen in neonates >14 days old.
As per manual method, ≥ 0.2 was considered as positive I/T ratio. The reference intervals for paediatric population in automated IGC and IG% were not well defined in literature. Therefore, adult reference ranges were considered, though the literature survey had highlighted two different cut off values for IGC (>0.03 and 0.3) and IG% (>0.5% and >3%) [8].
Sensitivity and Negative Predictive Value (NPV) were calculated as the two key performance Characteristics.
Ethical Consideration
FBC analysis, blood culture and CRP consisted part of the investigations in a diagnostic work up of a baby with suspected sepsis. For other babies who were admitted to the neonatal unit for various reasons, FBC analysis was a routine test. Therefore, there were no additional procedures done on the patient to obtain samples. However, ethical clearance for the study was obtained from the Ethical Review Committee, Colombo South Teaching Hospital prior to starting the study. Mother of each neonate was informed about the study and verbal consent was taken for inclusion.
There was no involvement of the patients or members of the public in the design, or conduct, or reporting, or dissemination plans of the research
Results
Within the study period, 51 babies were included, and there were 14 neonates with <34 weeks of age (extreme premature), 9 were between 34-37 weeks and the rest were term babies.
In the study group of 51 neonates, 45 babies had septic screen and six (6) had FBC for other reasons e.g. jaundice due to haemolysis. However out of 45 who had septic screen, only 39 neonates were started on antibiotics for suspected sepsis. Finally, 19 were diagnosed with proven sepsis including 18 culture negative probable sepsis and one culture positive definitive sepsis. It was highlighted that there was only one positive blood culture reported in this population group, which was 2.6% of treated population (1/39) and 5.3% of proven sepsis (1/19) TABLE 1.
TABLE 1. Distribution of the sample according to the diagnosis.
| Category | Number | Percentage |
|---|---|---|
| FBC done for other reasons | 6 | 11.80% |
| Investigated for sepsis, but not treated with Antibiotics | 6 | 11.80% |
| Investigated and treated with antibiotics, no proven sepsis | 20 | 39.20% |
| Culture negative proven sepsis | 18 | 35.30% |
| Culture positive sepsis | 1 | 1.90% |
| Total | 51 | 100% |
Out of the 39 neonates who were initially treated with antibiotics, 20 patients (51.3%) who became negative in septic screen later and were discontinued on antibiotics.
A total of 42 samples were analyzed for manual I/T ratio (11+31) and 40 samples by automated IGC and IG% (31+09) according to the data given in TABLE 2.
TABLE 2. Number of samples analyzed for manual I/T, automated IGC and IG%.
| Mode of analysis | Number |
|---|---|
| Manual I/T ratio only | 11 |
| Both manual I/T ratio and automated IGC and IG% | 31 |
| Automated IGC and IG% only | 9 |
| Total | 51 |
Out of the 42 samples analyzed for manual I/T ratio, 21 had I/T ratio of ≥ 0.2 (positive). Among them 15 were finally diagnosed with sepsis (TP) and 6 were negative (FP) TABLE 3. The sensitivity and NPV for the manual I/T ratio were 93.8% and 95.2% respectively. Five out of six of those false positive babies were extremely premature (<34 weeks of POA) and one term baby but with congenital kidney disease.
TABLE 3. I/T ratio for manual method.
| Final Diagnosis\I/T ratio | Sepsis | No sepsis | Total |
|---|---|---|---|
| Positive (≥ 0.2) | 15 (TP) | 06 (FP) | 21 |
| Negative (<0.2) | 01 (FN) | 20 (TN) | 21 |
| Total | 16 | 26 | 42 |
Forty (40) out of 51 samples were analyzed for automated IGC with the cut-off value of 0.03 TABLE 4 and 0.3, TABLE 5 giving different results. The sensitivity for the two cut off values were 80% and 40% respectively. In contrast, the NPV rose from 25% to 70.9 % with IGC of 0.3.
TABLE 4. Automated IGC for cut-off 0.03.
| Final Diagnosis\IGC (0.03) | Sepsis | No sepsis | Total |
|---|---|---|---|
| Positive (>0.03) | 12 (TP) | 24 (FP) | 36 |
| Negative (≤ 0.03) | 03 (FN) | 01 (TN) | 4 |
| Total | 15 | 25 | 40 |
TABLE 5. Automated IGC for the cut-off of 0.3.
| Final Diagnosis\IGC (0.3) | Sepsis | No sepsis | Total |
|---|---|---|---|
| Positive (>0.3) | 06 (TP) | 03 (FP) | 9 |
| Negative (≤0.3) | 09 (FN) | 22 (TN) | 31 |
| Total | 15 | 25 | 40 |
Similarly, for IG% in automated method, the sensitivity at cut off of 0.5% was 73.3% and the NPV was 42.9%. When the cut-off was raised up to 3%, the sensitivity was 33.3% and NPV was 70.6% TABLE 6 and TABLE 7.
Table 6. Automated IG% cut-off value 0.5%.
| Final Diagnosis\IG% (0.5%) | Sepsis | No sepsis | Total |
|---|---|---|---|
| Positive (>0.5%) | 11 (TP) | 22 (FP) | 33 |
| Negative (≤ 0.5%) | 04 (FN) | 03 (TN) | 7 |
| Total | 15 | 25 | 40 |
Table 7. Automated IG% for the cut-off 3%.
| Diagnosis\IG% (3%) | Sepsis | No sepsis | Total |
|---|---|---|---|
| Positive (>3%) | 05 (TP) | 01 (FP) | 6 |
| Negative (≤ 3%) | 10 (FN) | 24 (TN) | 34 |
| Total | 15 | 25 | 40 |
Discussion
As mentioned earlier, diagnosis of neonatal sepsis is challenging due to the non-specific and subtle nature of the clinical picture. In routine practice, there is no single laboratory parameter that is used to diagnose neonatal sepsis. Therefore, high clinical suspicion with other laboratory parameters namely, positive blood culture, blood count parameters and positive CRP at 48 hours are used [10,11]. Out of the laboratory parameters, blood culture positivity is considered as the gold standard. However, this includes several drawbacks including low sensitivity which was also highlighted in the current study, due to inadequate sample volume from the neonates and improper sampling technique and long duration taken for the result (24 hours-48 hours) hence, delaying a prompt diagnosis of sepsis [1-5]. Although CRP is a rapid test, it will take about 24 hours for the neonatal body to produce a significant level of CRP [12].
All these factors lead to unnecessary exposure of healthy neonates who actually do not have sepsis, to antibiotics for minimum of three days. In the current study, the percentage of neonates who were initially started on antibiotics and then discontinued later as no proven sepsis was 51.3% (n=20/39).
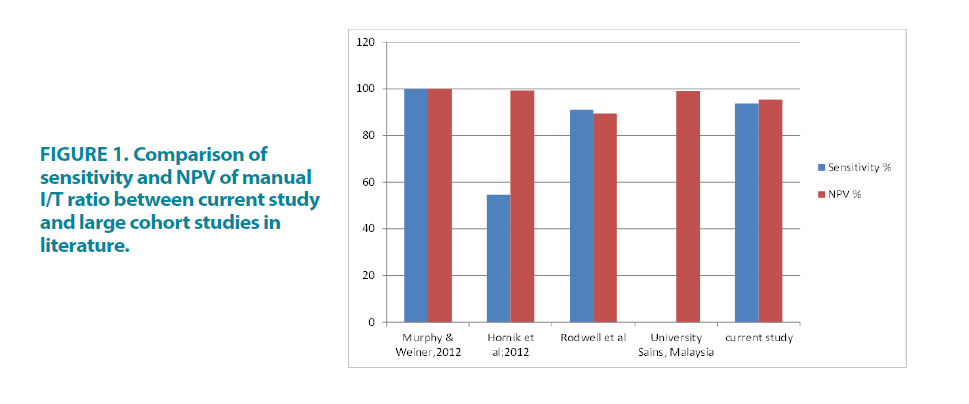
Immature to Total neutrophil (I/T) ratio by manual method had been recognized as a useful tool. However, it was not widely used as it was labour intensive. Several studies have proved that a low IT ratio of 0.2> has a very high negative predictive value (some close to 100%) and a good sensitivity rate [1-6]. The results in our study were reassuring with sensitivity of 93.8% and NPV of 95.2% touching the figures in larger cohort studies which indicated 98%- 100% FIGURE 1.
Considering above factors, it is clearly seen that manual I/T ratio is a good screening test to exclude early onset neonatal sepsis in combination with other clinical and laboratory parameters. It is still useful in premature babies as there were no false negatives found among premature babies in our study.
Innovations in technology leads automated analyzers in counting immature granulocytes based on light scattering and fluorescence techniques. This gives a solution for main drawbacks in manual I/T ratio such as labour cost, inter and intra observer variance in morphology.
However, the reference interval for pediatric age group in automated IGC and IG% was not well defined. Also in adult reference ranges, two different cut-offs for each were proposed.
In current study, sensitivity for cut off values, IGC 0.03 and IG% 0.5% were satisfactory with 80% and 73.3% respectively. Similarly in the study at Chettinad Deemed University, adults, children, infants and neonates, IGC of 0.03 × 103/μL and IG% of 0.5% offered a sensitivity of 86.3% and 92.2% respectively (82% and 89% in adults; 88% and 96% in children). Measurements were performed using the Coulter Act Diff 5 counter. Higher values of IGC >0.3 and IG% >3 had a specificity more than 90% although the values were infrequent. Data for neonates separately was not available in this study.
However, sensitivity for IG%, 0.5% was unacceptably low with 33% in study “Performance of immature granulocyte count as a predictor of neonatal sepsis” [8]. Due to the inconsistency and greatly varied sensitivity results given above, these cut-offs cannot be recommended.
Furthermore, in the current study the NPV for above cut-off values were very poor with 25% for IGC 0.03 and 42.9% for IG% 0.5% reflecting this as a poor predictor in excluding neonatal sepsis. The NPV was improved with higher cut-off values with 70.9% for IGC 0.3 and 70.6% for IG% 3%. Again the use of these cut-off values deemed unsuccessful with lower sensitivity of 40% and 33.3% respectively.
For automated IGC and IG% none of the above cut-off values have shown validity to use in diagnosis of early neonatal sepsis in current study or in literature. This points out the importance of further large cohort studies formulating specific reference ranges for neonates (or paediatric age groups), with proper validation and quality control.
Conclusion
Immature to Total neutrophil (I/T) ratio is a potentially useful adjunctive tool in the diagnosis and exclusion neonatal sepsis with a very good sensitivity and NPV. It is for use in conjunction with assessment of perinatal risk factors, clinical features and other laboratory investigations.
However, further studies with improvement in method of detecting immature granulocytes and well-defined reference intervals are required in automated IGC and IG%. As the sample size is small in our study, a future study with a larger cohort is highly recommended to draw better conclusions.
Acknowledgement
We would like to thank Dr. Rajiv Senthilnadajahal, Consultant Pediatrician and the staff in SCBU and Post-natal ward, Colombo South Teaching Hospital (CSTH), for the support given in data and sample collection, Ms.WMCW Manike, Mr. WATP Ranasinghe and other staff members at haematology laboratory, professorial unit, CSTH and the staff at Haematology and Microbiology laboratories at National Cancer Institute Maharagama (NCIM), for data collection and sample analysis.
We would also like to thank Dr. Sashikala Suresh, Consultant Hematologist and Dr. Samanmali Gunasekara, Consultant Microbiologist at NCIM for giving permission to analyze samples in the relevant automated machines.
We would also thank Analytical instruments (PVT) Ltd. for supporting with quality control material and reagents for the study.
Disclosure of Conflict of Interest
All authors declare no conflict of interest.
References
- Hornik CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count in early-onset neonatal sepsis. Pediatr Infect Dis J. 31, 799-802 (2012).
- Makkar M, Gupta C, Pathak R, et al. Performance evaluation of hematologic scoring system in early diagnosis of neonatal sepsis. J Clin Neonatol. 2, 25-29 (2013).
- Ghosh S, Mittal M, Jaganathan G. Early diagnosis of neonatal sepsis using a hematological scoring system. Indian J Med Sci. 55, 495-500 (2001).
- Narasimha A, Harendra Kumar ML. Significance of Hematological Scoring System (HSS) in early diagnosis of neonatal sepsis. Indian J Hematol Blood Transfus. 27, 14-17 (2011).
- Murphy K, Weiner J. Use of leukocyte counts in evaluation of early-onset neonatal sepsis. Pediatr Infect Dis J. 31, 16-19 (2012).
- Rodwell RL, Leslie AL, Tudehope DI. Early diagnosis of neonatal sepsis using a hematologic scoring system. J Pediatr. 112, 761-767 (1988).
- Kelly G, Nigro MD, MaryAnn O’Riordan, et al. Performance of an automated immature granulocyte count as a predictor of neonatal sepsis. Am J Clin Pathol. 123, 618-624 (2005).
- Senthilnayagam B, Kumar T, Sukumaran J, et al. Automated measurement of immature granulocytes: performance characteristics and utility in routine clinical practice. Patholog Res Int. 2012, 483670 (2012).
- Walliullah SM, Islam MN, Siddika M, et al. Role of micro-ESR and I/T ratio in the early diagnosis of neonatal sepsis. Mymensingh Med J. 18, 56-61 (2009).
- Walliullah SM, Islam MN, Siddika M, et al. Evaluation of simple hematological screen for early diagnosis of neonatal sepsis. Mymensingh Med J. 19, 41-47 (2010).
- Selimovic A, Skokic F, Selimovic Z, et al. The predictive values of total white bood count and differential count in the diagnosis of early-onset neonatal sepsis. Med Arh. 62, 205-210 (2008).
- Ahmed Z, Ghafoor T, Waqar T, et al. Diagnostic value of C-reactive protein and haematological parameters in neonatal sepsis. J Coll Physicians Surg Pak. 15, 152-156 (2005).