Review Article - Interventional Cardiology (2013) Volume 5, Issue 1
Defining the role of covered stents in aortoĂƒÂƒĂ‚Â¢ĂƒÂ‚Ă¢Â‚Â¬ĂƒÂ‚Ă¢Â€Âœiliac interventions
- Corresponding Author:
- Vinay Kumar
Endovascular Center, South Central Regional Medical Center, Laurel, MS 39440, USA
Tel: +1 601 433 1245
E-mail: vklaurel@yahoo.com
Abstract
Keywords
aorta;balloon angioplasty;covered stent;iliac arteryn;stent
Introduction
Atherosclerotic peripheral arterial disease affects 10–12% individuals above the age of 65 years in the USA. An estimated 12–14 million Americans suffer from atherosclerotic peripheral arterial disease and approximately one-third of these cases occur due to aorto–iliac occlusive disease (AIOD). Although surgical therapy is still considered as standard of care, endovascular stent placement has gained popularity and has largely replaced surgery due to excellent short-term results [1]. Bare-metal stents are currently approved and are available in balloonexpandable and self-expanding formats. Although, bare-metal stents perform better in the short term, long-term patency of bare-metal stents remains inferior to the surgical bypass, requiring reintervention or surgical bypass in the future. The cause of long-term failure of baremetal stents is often attributed to tissue–metal reaction, atheroma prolapse through the stent struts and neointimal growth with resultant restenosis or occlusion.
Covered stents are designed by incorporating the surface of bare-metal stents with layers of polytetrafloroethylene (PTFE) on one or both surfaces, thus preventing the direct contact of metal with vessel wall or blood. The idea of covered stents is based on the in vitro study by Rogers and Edelman, who demonstrated a reduction in neointimal growth in the stents covered with PTFE (Table 1) [2]. If such is the case, covered stents should enhance patency, resulting in better long-term outcomes. This article describes currently available therapies and the current status of covered stents in AIOD.
| Stent type (days) | Neointimal (µm)† | |
|---|---|---|
| Uncoated corrugated ring (28) | 72 ± 23 | |
| ePTFE + corrugated ring (28) | 18 ± 03* | |
| Uncoated slotted tube (28) | 93 ± 16 | |
| ePTFE + slotted tube (28) | 17 | ± 04** |
| ePTFE + slotted tube (56) | 13 | ± 02 |
†Neointimal growth was studied in 28 cases. Values are presented as mean ± neointimal hyperplasia. *p < 0.007 compared with uncoated corrugated ring; **p < 0.0001 compared with uncoated slotted tube. ePTFE: Expanded polytetrafloroethylene. Reproduced with permission from [2].
Table 1. In vitro comparison of neointimal growth between bare-metal stent and polytetrafloroethylene-covered stent.
Treatment strategies for AIOD
Surgical bypass with synthetic graft has remained the treatment of choice of AIOD since its inception [3]. The procedure is performed by bypassing the diseased segment with a synthetic graft. These grafts are anstomosed proximally to the aorta and distally to the iliac or femoral arteries, bypassing the lesions. In high-risk patients, extra-antomical bypass can be created such as axillo–femoral or femoro–femoral bypass. Extra-anatomical bypasses usually carry lower patency rates.
Balloon angioplasty, with or without stent placement, was offered as an alternative to the surgical bypass using balloon-expandable or self-expanding stents. Although several studies concluded that the primary patency rates of surgical procedures were superior to stenting [4,5], surgical reconstruction demonstrated high complication rates, especially in high-risk and elderly patients, with published complication rates ranging from 8.3 to 12.2%, and mortality rates of 3.3–4.4% [5].
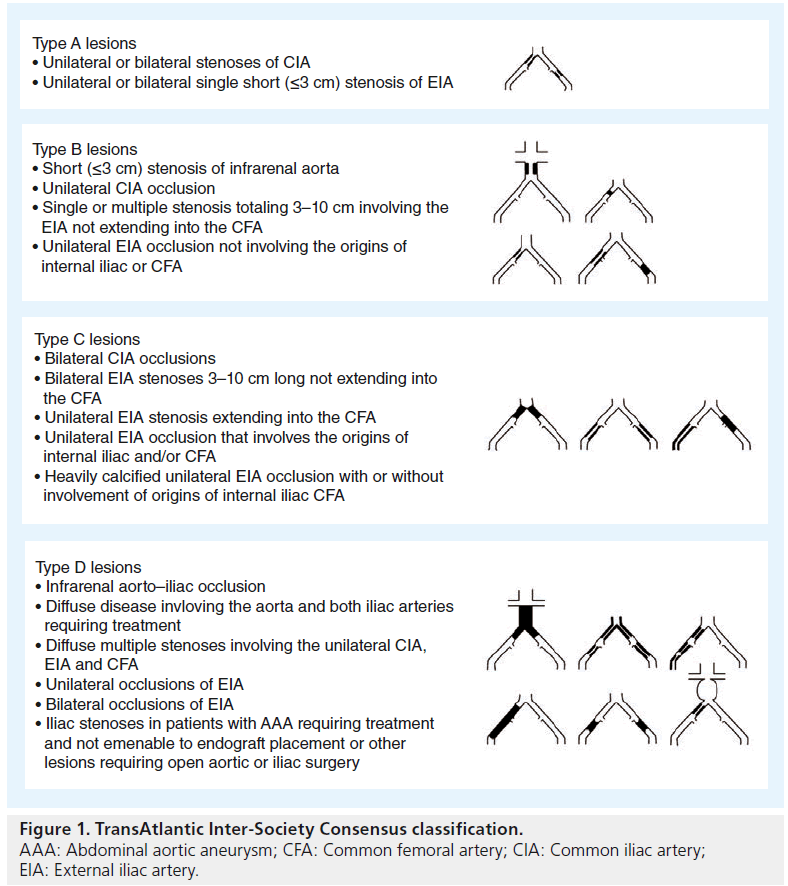
TransAtlantic Inter-Society Consensus recommendations
The TransAtlantic Inter-Society Consensus (TASC) classif ied lesions and offered recommendations for AIOD are based on currently available data [6]. TASC investigators recommended primary bare-metal stent placement for type A/B lesions, but cautioned against stents for type C/D lesions, since both patency and functional outcomes are worse in more diffusely diseased type C/D lesions (Figure 1).
This was confirmed by the BRAVISSIMO investigators, who conducted a prospective, nonrandomized, multicenter, multinational, monitored trial [7]. A total of 190 patients with TASC A/B lesions underwent primary bare-metal stent placement. At 12-months the primary patency rate was 94.0% for TASC A lesions and 96.5% for TASC B lesions.
Investigators at Yale University (CT, USA) reviewed 4119 patients who received treatment for AIOD from 2004 to 2007 [8]. In a bivariate analysis, endovascular procedures were associated with lower complication rates (16 vs 25%), shorter length of stay (2.2 vs 5.8 days), and lower hospital costs (US$13,661 vs 17,161) compared with open surgical procedures.
Primary stenting has proven to be advantageous for AIOD lesions than a more conservative selective stenting strategy [8–10]. In a recent analysis by Jonakind et al., 19 nonrandomized cohort studies with 1711 patients were evaluated for extensive AIOD. 4–5-year primary and secondary patency rates ranged from 60 to 86%, and 80 to 98%, respectively [11]. The authors concluded that endovascular treatment of extensive AIOD can be performed successfully by experienced operators in selected patients. Although, primary patency rates were lower than those reported for surgical revascularization, they concluded that reinterventions can often be performed percutaneously, with secondary patency comparable with surgical repair.
Although TASC recommends a surgical approach for more complex and TASC C/D lesions, due to the availability of a variety of chronic total occlusion devices and re-entry tools, enthusiasm to treat complex lesions is on the rise. Intervention of such lesions and treatment with bare-metal stents produces inferior results and unacceptable patency. In addition, patients who are more likely to have significantly poorer outcome and high reintervention rates include females, smokers, diabetes mellitus sufferers and those aged less than 50 years. Lesion characteristics include chronic total occlusion, poor run off, severe diffuse disease and heavily calcified lesions of bifurcation, needing kissing stents [12,13].
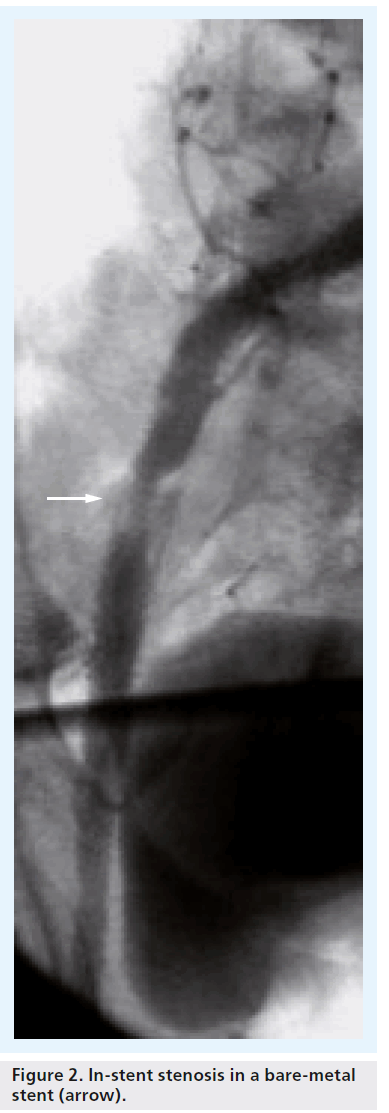
The major issue related to bare-metal stent placement is restenosis and subsequent occlusion. In complex iliac disease, treatment of restenosis can be very challenging, with potential intervention complications such as recurrence, thrombosis, dissection and distal embolization. Simply placing a stent in a lesion does not cure the disease, and patency rates may be influenced by neointimal tissue in-growth [14], potentially leading to poorer clinical outcomes [12], and higher reintervention rates (Figure 2).
Figure 2: In-stent stenosis in a bare-metal stent (arrow).
Patient demographics & lesion characteristics
A recent meta-analysis of 16 published studies with 958 patients was analyzed to determine the technical success and long-term patency of TASC C and D aorto–iliac arterial lesions undergoing primary or selective stenting [15]. Subgroup analyses demonstrated that the technical success rate for TASC C and D lesions for primary stenting was 94.2 and 92.1%, respectively, and patency at 12 months was 88.0 and 82.9%, respectively. The authors concluded that long-term, primary patency rates for patients receiving primary stenting were significantly better than those receiving selective stenting.
Where do covered stents fit
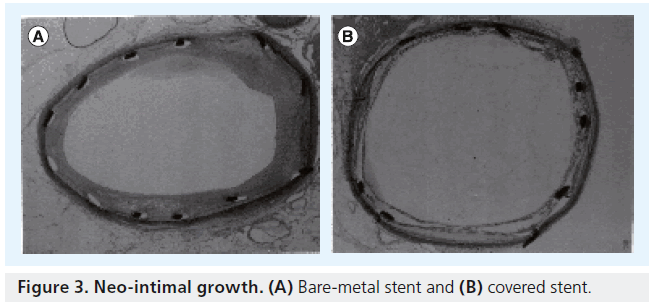
Our goal then should be to achieve long-term patency of stents in iliac arteries, irrespective of TASC classification, and match endovascular results to surgical bypass. An alternative strategy that may be considered in the AIOD patient is the placement of covered stent technology in these complex lesions. Rogers and Edelman performed an in vitro comparison of neointimal growth between bare-metal balloon-expandable stents and PTFE-covered balloon-expandable stents in rabbit iliac arteries [2]. They concluded that “PTFEcovered stents may protect against the atheroma prolapsing, and in addition, may help prevent neointimal hyperplasia” (Figure 3). By excluding the plaque, and encapsulating the disease, tissue can be prevented from extruding through the open stent struts during deployment, unlike a bare-metal stent (Figure 4), thus lessening the risk of restenosis and potentially reducing late lumen loss. In addition, the study also demonstrated early and late reduction in neointimal growth, which will further reduce restenosis.
„„▪ Covered stents
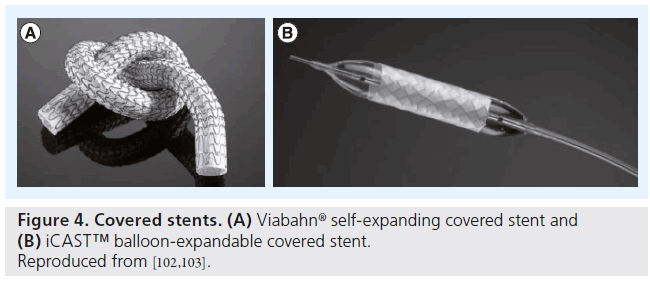
Currently there are two covered stents available, each with unique properties. The iCAST™ or V12 (both Atrium Medical Corporation, NH, USA) is a balloon-expandable stent designed with a slotted stainless steel tube that is completely encapsulated in a microporous thermo-conformable PTFE. The stent is available in a variety of sizes and is supplied mounted on a noncompliant patented balloon. The manufacturer also utilizes a powercrimp technology that prevents slippage of the covered stent. It is 7 F sheath compatible and deployed over a 0.35-inch wire.
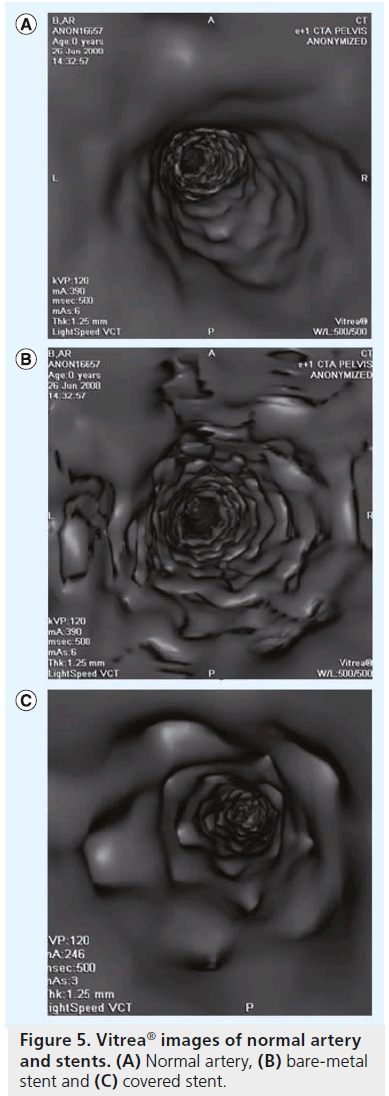
The Viabahn® stent graft (Gore Medical, AZ, USA), on the other hand, is constructed with a durable, reinforced, biocompatible-expanded PTFE liner attached to an external nitinol stent structure. The stent graft is deployed via 7–12 F sheath using a unique pull cord delivery system. It is extremely flexible and conforms well to the vessel wall and the proximal end of the stent graft is scalloped to prevent in-folding; however, it does lack the radial strength of a balloon-expandable stent (Figure 5).
Covered stent publications in AIOD
Several studies have demonstrated that PTFEcovered stents may provide better results as compared with bare-metal stents, particularly in TASC C and D lesions. A recent meta-analysis by Grimme et al. completed a literature review of 51 articles for bare-metal and covered stents, where 21 articles on bare-metal stents (selective and primary use, mostly TASC A and B lesions) and three articles on the primary use of covered stents were utilized [16]. The authors concluded that patients who received bare-metal stents had significantly lower primary patency rates at 1 (78%), 2 (72%) and 4 years (53%), whereas patients treated with a balloon-expandable covered stent were more likely to remain free from restenosis at 18 months. The authors concluded that “the randomized trials had proven the superiority of covered stents in extensive iliac occlusion lesions”.
Between 1997 and 2006, Chang et al. retrospectively reviewed 171 patients (22 bilateral patients) who underwent 193 common femoral artery endarterectomies with patch angioplasty and primary stenting or stent grafting in a combined hybrid open and endovascular procedure for treatment of TASC C and D iliofemoral occlusive disease [17]. Covered stents were utilized in 41% of the patients. At 5 years, significantly higher patency rates were seen in patients receiving covered stents as compared with bare-metal stents when used to treat TASC C & D common and external iliac lesions (87 vs 53%; p ≤ 0.01).
Kissing stents
An early bare-metal stent series from Haulon et al. reviewed 106 patients treated with kissing stents [18]. Technical success was 100%, with no major procedure-related complications. Self-expanding stents were placed in 62 patients (58.5%), and balloon-expandable stents in 44 patients (41.5%). The average follow-up was 30.1 ± 11.1 months (range: 12–137 months). Duplex imaging showed restenosis in 15 (14.8%) and occlusion in 4 (4%). Primary patency at 36 months was 79.4%.
Greiner et al. evaluated 41 patients with kissing stents for primary and assisted patency over an 8-year period (1997–2005) [19]. Patients were divided into two groups. A noncrossing group (defined as the proximal ends of the stents overlapped half of their length or less within the aorta) versus the ‘crossing group’ (defined as the proximal end of the kissing stents overlapped more than half of their length within the aorta). The primary and assisted primary patency rates at 2 years were significantly different (p = 0.01) with noncrossing stents, showing a 94.1% primary patency versus 33.2% patency in the crossing group. Assisted patency for the noncrossing group was 100% versus the crossing group at 45.3%. This may be explained by the presence of thrombus formation created by intimal hyperplasia, due to stagnant blood flow, created by the presence of a vascular space outside of the kissing stent space [20,21].
A case series from the University of Virginia (VA, USA) sought to investigate their outcomes of balloon-expandable covered stents in the kissing stent position [22]. They retrospectively reviewed patients who received Atrium iCAST balloon-expandable covered kissing stents (n = 26) versus bare-metal kissing stents (n = 28) for the treatment of aorto–iliac lesions. Technical success was achieved in 100% of patients in both groups. Complications occurred in three patients receiving covered stents, and two patients receiving bare-metal stents (p = 0.66). Average follow-up was 21 months (20 months for covered stents vs 25 months for bare-metal stents; range: 1–62 months). Patients who were treated with covered stents had sustained improvement in clinical symptoms (85%) during the follow-up period compared with bare-metal stents (54%; p = 0.02). Primary patency rates at 1 and 2 years were both 92%, respectively, for covered stents and 78% and 62% for bare-metal stents (p = 0.023). Of note, the covered stent lesion characteristics were statistically more advanced than those in the bare-metal group (38% of the covered stent group had type C & D lesions versus 7% for bare-metal stent; p = 0.034). The covered stent group had superior outcomes, regardless of the type of lesions treated. The recently published COBEST trial was a prospective, multicenter, randomized controlled trial, which compared the Atrium V12 balloonexpandable covered stent with bare-metal stents for iliac disease [23]. The primary end point of the study was the rate of binary restenosis (defined by 50% reduction in lumen diameter) and freedom from stent occlusion at 18 months. Patients were assessed clinically with an ankle-brachial index (ABI) and aorto–iliac arterial Duplex ultrasound at intervals of 1, 6, 12 and 18 months. The use of balloon-expandable covered stents demonstrated superiority to bare-metal stents at 18-month follow-up, with lower rates of binary restenosis (p = 0.03), freedom from stent occlusion (p = 0.04) and a four-times lower target-lesion revascularization rate compared with bare-metal stents (p ≤ 0.05) [15]. This superiority in outcomes was most profound in TASC C and D lesions.
Investigators of the COBEST trial observed that the restenosis occurred at the edge of stents, at the level of stent–vessel contact, not in the middle of the stent as is most commonly seen with bare-metal stents and recommended that stents should be placed across the lesion, from normal vessel above to normal vessel below.
The authors have tracked all of the patients receiving covered stents during intervention of aorto–iliac lesions in our practice since 2004. A retrospective analysis was conducted on 174 patients (59 male, average age 72 ± 33 years) in 201 limbs (71 bilateral kissing stents) who received balloon-expandable covered stents (iCAST, Atrium Medical Corporation) for intervention of the iliac artery(s). In total, 22 patients received computed tomography angiography follow-up using Vitrea ® software (Figure 5), which was reviewed independently by an interventional radiologist, not involved in the initial treatment. All patients received clinical follow-up with a lifestyle questionnaire and ABIs every 6 months. The average long-term follow-up was 43 months (range: 6–84 months).
Immediate technical success was 100%, with no thrombosis or stent-related complications. There was one access site complication related to the closure device. The average lesion length was 29 mm (range: 10–64 mm), and 30% of the lesions treated were chronic total occlusions. A total of 11% of the lesions were utilized for the treatment of in-stent restenosis of previously placed bare-metal stents. During the follow-up period, 38 patients died of causes unrelated to the procedure or device. Late occlusion occurred in two patients (27 and 29 months, respectively) and there were no amputations. Average ABI at 43 months was 0.7 (0.8–1.2). Lower ABI mainly occurred due to multilevel disease in several of these patients.
In our retrospective series, it was observed that covered stents are safe and are a superior alternative to bare-metal stents. This is demonstrated by the clinical patency of 99.4% at 6–76 months (mean 46 months) follow-up and freedom from amputation in all patients. The use of balloon-expandable PTFE-covered stents in iliac arteries significantly reduces reinterventions, and appears to result in better long-term outcomes in comparison with bare-metal stents.
The data of the author of this review is very similar to Bosiers et al., one of the first publications with balloon-expandable covered stents [24]. In their nonrandomized trial at two centers, 91 limbs in 65 patients were treated for iliac artery occlusive disease and claudication with no procedural complications. A total of 50% of lesions were heavily calcified, including eight total occlusions with an average lesion length of 41.2 mm. This nonrandomized, prospective study found that the mean ABI rose significantly from 0.59 before treatment, to 0.98, 0.98 and 0.99 at 1, 6 and 12 months,respectively, after the procedure. Primary limb patency at 1-year was 91.1%.
Conclusion
The idea behind utilization of covered stents is to achieve long-term primary patency similar to surgical therapy; however, none of the randomized studies have published long-term results. Current published data show the superiority of covered stents over bare-metal stents in short- and mid-term follow-up. The COBEST trial is a well-designed randomized trial, but their total follow-up has been 18 months. Although the results in TASC A and B groups were similar to bare-metal stents, better outcomes were demonstrated for TASC C and D lesions. Their results are promising; however, more randomized data are needed to ascertain long-term patency. Our unpublished series demonstrated patency in all lesions to be 99.6% over 46 months and also demonstrated no significant difference based on TASC classification. While results of the ICARUS trial are awaited, it seems that the best objective data is from Chang et al. [17]. Authors have carefully followed their patients during hybrid procedures of femoral endarterectomy combined with iliac angioplasty with bare-metal or covered balloon-expandable stents. In their long-term follow-up, covered stents demonstrated significantly better patency in comparison with bare-metal stents (87 vs 53%).
In conclusion, based on current publications and our own experience, covered stents are strongly thought to be beneficial in the treatment of complex aorto–iliac disease, especially chronic total occlusions, aorto–iliac stenosis (needing kissing stents) and younger patients where longterm patency is a definite issue with bare-metal stents.
Future perspective
As we await with anticipation the results of the ICARUS trial [101], early data presented by John Laired during the VIVA 2012 (Vascular Interventional Advances) meeting in Las Vegas, USA is very encouraging. ICARUS is a prospective, multicenter clinical trial, involving 165 patients across 25 centers, that will shed more light on the efficacy and long-term patency of covered stents in comparison to bare-metal stents. The study has met its endpoint and at 9-month follow-up, target lesion revascularization was only 2.9%, indicating excellent short-term patency; detailed data is expected in 2014. Based on the previously published reports, it the general feeling that this trial will also establish superiority of covered stents in aorto–iliac disease.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
ƒƒ Atherosclerotic peripheral arterial disease of the aorto–iliac segment constitutes approximately one-third of all cases needing revascularization. Surgical therapy is the standard of therapy due to its long-term durability. In recent years, endovascular stent placement has gained popularity and currently available data indicate that bare-metal stents perform better in the short term, but longterm patency is inferior to open surgical procedures. Covered stents have been introduced to improve long-term patency. This idea is based on the use of a polytetrafloroethylene (PTFE) covering over a bare-metal stent that has shown reductions in neointimal growth and improved patency in vitro. It is the general feeling that a PTFE-covered stent may improve long-term patency in aorto–iliac occlusive disease (AOID).
Treatment strategies
ƒƒ Surgery and endovascular stent placement have their own benefits and pitfalls. Although surgical bypass with synthetic grafts produces better long-term patency, complications and mortality are significantly high as compared with the endovascular group. Endovascular stent placement, on the other hand, is associated with higher reintervention rates.
TransAtlantic Inter-Society Consensus recommendations
ƒƒ TransAtlantic Inter-Society Consensus (TASC) recommendations are based on currently available data. Society recommends endovascular therapy for TASC A and B lesions and surgical therapy for TASC C and D lesions.
Where do covered stents fit?
ƒƒ Covered stents are designed with PTFE coating on the surface and are available in both balloon-expandable and self-expanding formats. PTFE covers the atheroma, prevents prolapse and reduces neointimal growth, thus lessening the risk of restenosis and occlusions.
Covered stent publications in AIOD
ƒƒ Multiple publications have compared the results of bare-metal stents and covered stents in AIOD. Results are published based on TASC criterion. Although patency rates are better in all-comers, the most notable differences are observed in TASC C and D lesions. Even in short-term follow-up in complex aorto–iliac lesions, such as chronic total occlusion and lesions needing kissing stents, improved patency was observed (COBEST trial, Chang et al., Haulon et al. and Greiner et al.). Our unpublished data also support their findings.
Conclusion
ƒƒ Covered stents are definitely superior to bare-metal stents in the treatment of AOID. Data support their use to obtain long-term patency especially in complex lesions (TASC C and D).
Future perspective
ƒƒ The US randomized trial ICARUS has finished enrolling and results are awaiting.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Palmaz JC, Laborde JC, Rivera FJ, Encarnacion CE, Lutz JD, Moss JG. Stenting of the iliac arteries with Palmaz stent: experience from a multicenter trial. Cardiovasc. Intervent. Radiol. 15(5), 291–297 (1992).
- Rogers C, Tseng DY, Gingras PH, Karwoski T, Martakos P, Edelman ER. Expanded polytetrafluorethylene stent graft encapsulation reduces intimal thickening regardless of stent design. J. Am. Coll. Cardiol. 31, 1163–1180 (1998).
- Malone JM, Moore WS, Goldstone J. The natural history of bilateral aortofemoral bypass grafts for ischemia of the lower extremities. Arch. Surg. 110(11), 1300–1306 (1975).
- Ballard JL, Bergan JJ,Singh P, Yonemoto H, Killeen JD. Aortoiliac stent deployment versus surgical reconstruction: analysis of outcome and cost. J. Vasc. Surg. 28, 94–103 (1998).
- De Vries SO, Hunink MG. Results of aortic bifurcation grafts for aortoiliac occlusive disease: a meta-analysis. J. Vasc. Surg. 26, 558–569 (1997).
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 45(Suppl. S), S5–S67 (2007).
- Bosiers M, Deloose K, Callaert J, Verbist J, Keirse K, Peeters P. BRAVISSIMO study: 12-month results from the TASC A/B subgroup. J. Cardiovasc. Surg. (Torino) 53(1), 91–99 (2012).
- Indes JE, Mandawat A, Tuggle CT, Muhs B, Sosa JA. Endovascular procedures for aorto– iliac occlusive disease are associated with superior short-term clinical and economic outcomes compared with open surgery in the inpatient population. J. Vasc. Surg. 52(5), 1173–1179 (2010).
- Kashyap, VS, Pavkov ML, Bena JF et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. J. Vasc. Surg. 48, 1451–1457 (2008).<
- AbuRahma A, Hayes D, Flaherty S, Peery W. Primary iliac stenting versus transluminal angioplasty with selective stenting. J. Vasc. Surg. 46(5), 965–970 (2007).
- Rogers C, Tseng DY, Gingras PH, Karwoski T, Martakos P, Edelman ER. Expanded polytetrafluorethylene stent graft encapsulation reduces intimal thickening regardless of stent design. J. Am. Coll. Card. 31, Abstract 1163–1180 (1998).
- Jongkind V, Akkersdijk GJ, Yeung KK, Wisselink W. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J. Vasc. Surg. 52(5), 1376–1383 (2010).
- Davies MG, Bismuth J, Saad WE, Naoum JJ, Peden EK, Lumsden AB. Outcomes of reintervention for recurrent disease after percutaneous iliac angioplasty and stenting. J. Endovasc. Ther. 18(2), 169–180 (2011).
- Timaran CH, Prault TL, Freeman MB, Goldman MH. Iliac artery stenting versus surgical reconstruction for TASC type B and type C iliac lesions. J. Vasc. Surg. 38(2), 272–278 (2003).
- Ye W, Liu CW, Ricco JB, Mani K, Zeng R, Jiang J. Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto–iliac lesions. J. Vasc. Surg. 53, 1728–1737 (2011).
- Grimme FAB, Goverde PA, Van Oostayen JA, Zeebregts CJ, Reijnen MMPJ. Covered stents for aortoiliac reconstruction of chronic occlusive lesions. J. Cardiovasc. Surg. 53, 279–289 (2012).
- Chang R, Goodney P, Baek J, Nolan B, Rzucidlo E, Powell R. Long-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive disease. J. Vasc. Surg. 48(2) 362–367 (2008).
- Haulon S. Percutaneous reconstruction of the aortoiliac bifurcation with the ‘kissing stents’ technique: long-term follow-up in 106 patients. J. Endovasc. Ther. 9, 363(2002).
- Greiner A, Mühlthaler H, Neuhauser B et al. Does stent overlap influence the patency rate of aortoiliac kissing stents? J. Endovas. Ther. 12, 696–703 (2005).
- Yilmaz S, Sindel T, Golbasi I, Turkay C, Mete A, Lüleci E. Aortoiliac kissing stents: long-term results and analysis of risk factors affecting patency. J. Endovasc. Ther. 13(3), 291–301 (2006).
- Saker MB, Oppat WF, Kent SA et al. Early failure of aortoiliac kissing stents: histopathologic correlation. J. Vasc. Interv. Radiol. 11, 333–336 (2000).
- Sabri S, Choudhri A, Orgera G et al. Outcomes of covered kissing stent placement compared with bare metal stent placement in the treatment of atherosclerotic occlusive disease at the aortic bifurcation. J. Vasc. Interv. Radiol. 21(7) 995–1003 (2010).
- Mwipatayi P, Thomas S, Wong J et al. A comparison of covered versus bare expandable stents for the treatment of aortoiliac occlusive disease (COBEST). J. Vasc. Surg. 54(6), 1561–1570 (2011).
- Bosiers M, Iyer V, Deloose K, Verbist J, Peeters P. Flemish experience using the Advanta V12 stent-graft for the treatment of iliac artery occlusive disease. J. Cardiovasc. Surg. (Torino) 48(1), 7–12 (2007).
- Atrium iCAST Iliac Stent Pivotal Study (iCARUS). http://clinicaltrials.gov/ct2/show/ NCT00593385?term=ICARUS&rank=3
- GORE® VIABAHN® Endoprosthesis. www.goremedical.com/viabahn/
- iCAST™ Covered Stent. www.atriummed.com/EN/interventional/ icast.asp
▪ Well-designed trial, shows efficacy of bare-metal stent in TransAtlantic Inter-Society Consensus A and B lesions.
▪▪ Vascular surgery prospective on surgical and endovascular therapy, and the benefits and shortcomings of each therapy.
▪▪ Presents an unbiased analysis of bare-metal and covered stents, concluded with the superiority of covered stents in complex lesions.
▪▪ Interesting paper as the authors have used combined surgical endarterectomy for the femoral artery with endovascular stent placement for inflow. Covered stents demonstrated better patency in their series.
▪▪ Data from a randomized trial, with emphasis on the use of covered stents in TransAtlantic Inter-Society Consensus C and D lesions.