Research Article - Neuropsychiatry (2019) Volume 9, Issue 2
Effects of Neural Stem Cell Transplantation on Differentiation of Dopaminergic Neurons in Substantia Nigra and Rotational Behavior Changes of Parkinson's Disease Rats
- Corresponding Author:
- Xiao-Ping Wang
Department of Neurology, Shanghai Tong-Ren Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200336, China
Tel: +86-21-63240090
Fax: +86-21-63243755
Abstract
Objective
To explore the effects of neural stem cells (NSCs) transplantation on the differentiation of
substantia nigra (SN) dopaminergic neurons and the rotational behavior on rat model of
Parkinson’s disease (PD).
Methods
Adult SD rats were randomly split into three groups of five: control, sham and transplanted
groups. 6-OHDA (2 μg/μl) was microinjected (8 μl) into the right medial forebrain bundle
(MFB) of sham and transplanted rats. Rats in the transplanted group were injected with 5 μl
NSCs suspension (5 × 104 cells/μl) into the right SN while an equal volume of PBS solution was
injected in the sham group.
Results
Eight weeks after transplantation, Tyrosine hydroxylase (TH-ir) neurons presented slight
somata and few dendrites, the levels of cell counting, protein synthesis and mRNA expression
were significantly decreased in both the sham and transplanted groups (p¼0.05). However,
compared with the sham group, the levels in the transplanted group increased (p¼0.05). Two
weeks after transplantation, the rotational behavior in the transplanted group significantly
was improved compared to pre-transplantation; this was also significantly different in the
eighth week (p¼0.05), and there were no significant improvement differences in the rotational
behavior in the sham group.
Conclusion
Transplanted NSCs are able to differentiate into SN dopaminergic neurons and
attenuate characteristic behaviors in rat model of PD.
Keywords
Parkinson’s disease, Neural stem cells, Substantia nigra, Tyrosine hydroxylase, Rotational behavior
Introduction
Parkinson’s disease (PD) is second to Alzheimer’s disease, as the most common progressive neurodegenerative disease. It is characterized by the degeneration of dopaminergic neurons in the substantia nigra coincident with progressive depletion of dopamine in the striatum [1]. At present, the main treatments for PD are medication and functional surgery [2,3], however as the period of treatment prolongs the efficacy reduces sharply with increased complications. The current treatment approaches do not prohibit the progression of PD when the diffuse lost neurons of substantia nigra in the brain area are not supplemented. The causes of PD are not fully elucidated. Patients are commonly diagnosed in the middle to late stages and already have severe symptoms when seeking medical help. As such, treatment with the currently symptom-targeting or modifying treatments available does not result in long-term optimal outcomes.
Application of neural stem cells (NSCs) as a potentially alternative therapy is being tested in the hopes of reducing the occurrence of severe complications by regenerating lost neurons. An optimal PD treatment would restore the dopamine content in the brain and also revive the functionality of the nigra-striatal pathway. To date, treatment with endogenous NSCs has been used as an alternative treatment in adult human and primate brain and primate brain (particularly in the hippocampus) have demonstrated limited capacity to survive, differentiate and mature and this has limited the development of the application of endogenous NSCs as a viable treatment. The ever-developing techniques in stem cell isolation and culture technology in vitro as well as advances in cerebral stereotaxic technology have brought new hope for attenuating the PD nigra-striatal pathway degeneration through NSCs transplantation [6,7]. In vitro culture and in vivo transplantation have shown that NSCs are able to markedly increase the expression of neurotropic factors such as nerve growth factor (NGF), brain derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3). This not only helps the transplanted NSCs to adapt well to the ischemic and/or hypoxia microenvironment, but also to protect the remaining lesioned endogenous cells [8,9]. The transplanted NSCs in the PD rat model striatum are able to survive and partly produce tyrosine hydroxylase (TH-ir) neurons. The newly generated neurons migrate to the SN and secrete dopamine that attenuates the PD rat rotational disorder, indicating that in vivo transplantation is likely to rebuild the neural network and the synaptic junctions [7,10,11]. Similar reports were already shown [11,12] .
Currently, there are few reports regarding NSCs injected into the substantia nigra (SN). Most of the depletion of PD dopaminergic neurons occurs in the substantia nigra, so this experiment aimed at study the effect of the NSCs injected into the SN on TH content. In the PD rat models this experiment would also assess the ability of transplanted NSCs to differentiate into dopaminergic neurons and alter rotational behaviors in a PD rat model. The results of these experiments may serve as a theoretical foundation to support the establishment of human clinical cell therapies for PD.
Materials and Methods
▪Materials
Sprague-Dawley (SD) rats (Slac Laboratory Animal Co., Ltd.) 6-dopamine,apomorphine (Sigma Company), rabbit anti-rat tyrosine hydroxylase antibody (Genetex Company), SABC diagnostic kit (WEIAO Biotech Co, LTD), DAB solution (Boster Biotechnology Company), MEM/F12 (Hyclone Company), B-27, FGF-Basic, EGF, Trypsin-EDTA (Gibco Company), adult rat stereotactic apparatus (WPI Company).
▪ PD Modeling
Fifteen healthy adult female SD rats, weighing 180-220 g, were randomly divided into 3 groups: PD model and sham group, PD model with NSCs transplant group, and control group. Rats in the first two groups were anesthetized with an abdominal injection of 10% chloral hydrate (5 ml/kg, i.p.) and immobilized in a stereotactic apparatus, with the incisors fastened by the dental rod and with the external auditory canals fastened by the ear bar. Then the dental rod was adjusted on the incisor surface to 2.4 mm lower than the ear bar. After the routine sterilization, the scalp was cut longitudinally along the centerline to find the Bregma. Using the Bregma as the origin of coordinates and the stereotactic apparatus map as the reference [12], two coordinates of the dextral medial forebrain bundle (MFB) were determined [13,14] antero-posterior (AP) 1.8 mm, medio-lateral (ML) 2.5 mm and dorso-ventral (DV) 7.5 mm; AP: 1.8 mm, ML: 2.5 mm and DV: 8.0 mm. The skull was drilled with the burr to expose the cranial cavity, and then 4 μl 6-OHDA (2 μg/μl) was microinjected at the depth of 7.5 mm with the microsyringe adjustor controlling the dripping speed, meanwhile avoiding the light (0.5 μl/min, for 5 min). After 4 μl 6-OHDA was injected, the needle remained for 5 minutes then went further to DV 8.0 mm with the same speed, and another 4 μl 6-OHDA was injected at the same rate. The needle was removed after the 10 min and the burr hole was enclosed with the glass-ionomer cement. Penicillin powder was sprinkled on the cutting surface and the opening was sutured after disinfection. The rats were labeled with the date and their general state. Two weeks after the drug administration, the rats received the hypodermic injection of apomorphine (1 mg/kg) at the nape of the neck to induce rotational tests. Revolutions per minute were counted for 30 min and rats that performed ≥ 7 r/min were regarded as successful PD modeling [15].
▪ NSCs Culture
Pregnant 14 d SD adult rats at 14 days gestation were anaesthetized and placed on an ultraclean bench. The mesencephalon midbrain of the fetal rats was dissected and the brain tissues were dissected and digested in trypsin with repeating blow through the suction tube. After digestion, the brain tissues were filtered through a 400-mesh cell screening. The filtrate was then centrifuged at 1000 rpm for 5 min. The remaining tissues (2 × 105 cells /ml) without the supernatant fluid were inoculated with the serum-free DMEM/F12 (4~5 ml) in the 50 ml culture flask, containing bFGF(20 μg/l), EGF(20 μg/l), B27(20 μg/l). When the cell refraction registered well from the microscopy observation, the tissues were transferred to the thermostat at 37℃ and 5% CO2. The growth of NSCs was monitored daily under an inverted microscope. The culture medium was changed every 2~3.5 days for a week or so for the passage culture.
▪ NSCs transplant
Anesthetized adult rats of the transplanted group were fastened to the stereotactic apparatus in order to determine the transplant coordinates of the NSCs in the SN [10]: AP 5.2 mm, ML 1.8 mm and DV 7.8 mm. The transplant site was microinjected with 5 μl NSCs suspension (5 × 105 cells/μl) that was cultured on the ultraclean bench at the rate of 1 μl/min. The needle remained for 10 mintues then was removed at the rate of 1mm/min. The sham group received iso-volumetric PBS solutions in place of NSCs suspension and all the other operation stayed the same.
▪ Behavioral experiment
Behavioral tests were performed at week 1, 2 weeks, 4 weeks and 8 weeks after the NSCs transplantation in the same quiet setting. The adult rats received a sub-dermal injection of APO (1 mg/kg) at the nape prior to rotational tests. The number of revolution within 30minutes was counted and the results from the transplanted group and the sham group were compared.
▪ Immunohistochemistry staining
Eight weeks after the NSCs transplantation,the SD rat brains of the two groups were harvested with 4% paraformaldehyde for fixation and immersed in 25% sucrose solutions for dehydration. The brain tissues were then cut on a freezing microtome for the coronal sections at the thickness of 10 μm. The coronal sections were rinsed by 3 times with PBS and subsequently steeped in the 0.6% hydrogen peroxide-methanol solution for 30 minutes at room temperature so as to inactivate the endogenous peroxidase vitality. After the second PBS rinsing,5% BSA (bovine serum albumin) sealing liquid was added to cover the sections for 20 minutes. When the liquid was absorbed by the filter paper, anti-rabbit tyrosine hydroxylase single clone antibody (1:1000 PBS dilution) was added to the sections and incubated at the temperature of 4℃ overnight; rinsed in PBS, then the secondary antibody biotin IgG, tri-anti streptomycin avidin biotin, and peroxidase enzyme were subsequently added and cultured for another 20 minutes at 37℃. After rinsing in PBS, the sections were placed at room temperature for 13min’s to develop DAB coloring. Three images of the substantial nigra pars compacta (SNpc) were chosen randomly under the light microscopy (x400 magnification) in order for the calculation of the positive cells [17].
▪Western blot
Total protein of the dextral rat nigral tissues was sampled in the Nonidet P-40 lysis buffer and then centrifuged for 15 minutes to extract the supernatant. The protein concentrations were tested in the bicinchoninic acid (BCA) proteinbinding assay kit (DGKC). An equal amount of protein sample was homogenized in the loading buffer and the total 10 μl mixture was treated in SDS-PAGE (80V for 30min then changed to 120V for over 60min). The protein separating gels were transferred to the nitrocellulose membrane before Ponceau staining. After the transmembrane success, the sample was blocked by 5% skim milk for 1 hour and then incubated with the anti-rabbit tyrosine hydroxylase single clone antibodies (1:2000 TBST buffer) at 4℃ for one night on the shaking table; washed with TBST and incubated with secondary anticochlearia peroxidase enzyme for 1 h at room temperature, labeled as goat anti-rabbit IgG. After the third rinse, enhanced chemiluminescence (ECL) reagents were added to expose the sample in a dark room for photography using gelimaging system. Densitometry of the banding strength was assessed through image acquisition and analysis software. Target proteins were contrasted with β-actin as reference, and the difference between the OD values indicated the expression level of target proteins.
▪ Real-time PCR
Total RNA was extracted from the adult rat dextral nigral tissues in TRIzol reagents and synthesized to cDNA by M-MLV reverse transcriptase. The Real-time PCR experiment was conducted with cDNA as the model. Primer sequences (Invitrogen company synthesis) are as shown in Table 1.
| Gene | Primer sequence (5’-3’) |
| TH[18] | Forward: AGC TGT GCA GCC CTA CCA AGA Reverse: GTG TGT ACG GGT CAA ACT TCA |
| β-actin |
Forward: CCT CTA TGC CAA CAC AGT Reverse: AGC CAC CAA TCC ACA CAG |
Table 1: Total RNA was extracted from the adult rat.
Amplification conditions: 94°C 5 min,94°C 30 s,57°C 30 s,72°C cycling 30 times/per min [18,19]. Afterwards, 2△△Ct method was applied to calculate the multiples of genic change between the transplanted group and the model group.
▪ Statistical analysis
All data were analyzed experimentally with the SPSS 19.0 statistical software and illustrated by Mean ± standard deviation, applying a one-way ANOVA for multi-group comparison and SNK test between two groups. A value of p<0.05 was considered as a statistically significant difference.
Results
▪ NSCs Culture
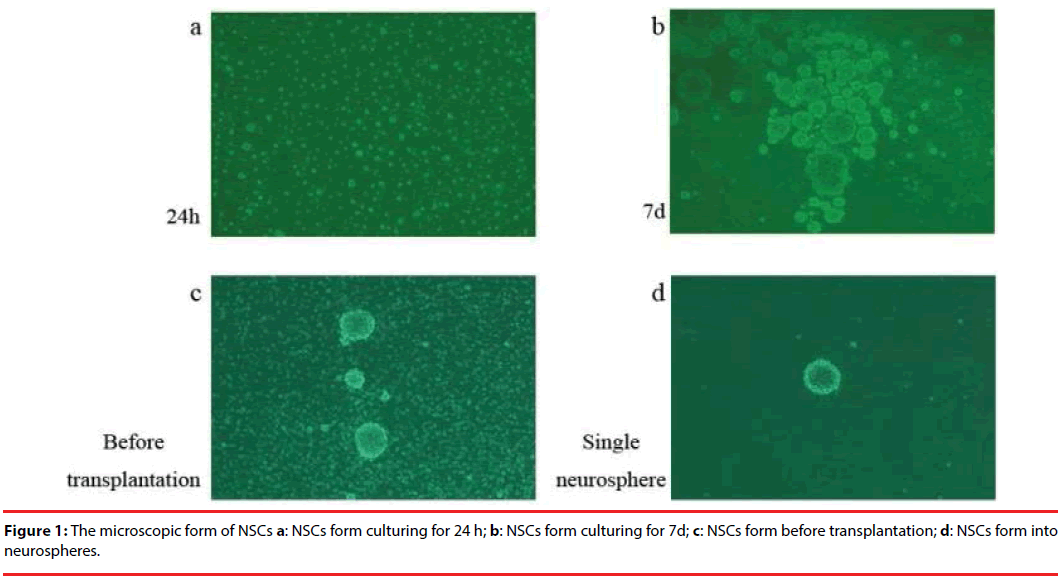
After 24 h of the original NSCs culture, stem cells expanded with the suspension growth, high refraction and high viability. Some of the cells were asymmetrically splitting and tiny neurospheres came into shape. When the culture reached the 7th day, neurospheres of different sizes appeared and bigger neurospheres showed weak refraction in the center but strong refraction around the edge, indicative of low cell vitality and deficit oxygen that impacted the nutrient supply and metabolism of the stem cells. This was the time to begin cell passages. Before the transplantation it was necessary to digest the neurospheres to avoid clogging the microsyringe needle and to reduce the risk of brain tumors. After the digestion, there are abundant single NSCs presented with just a few undigested neurospheres left (Figure 1).
▪ Behavioral tests
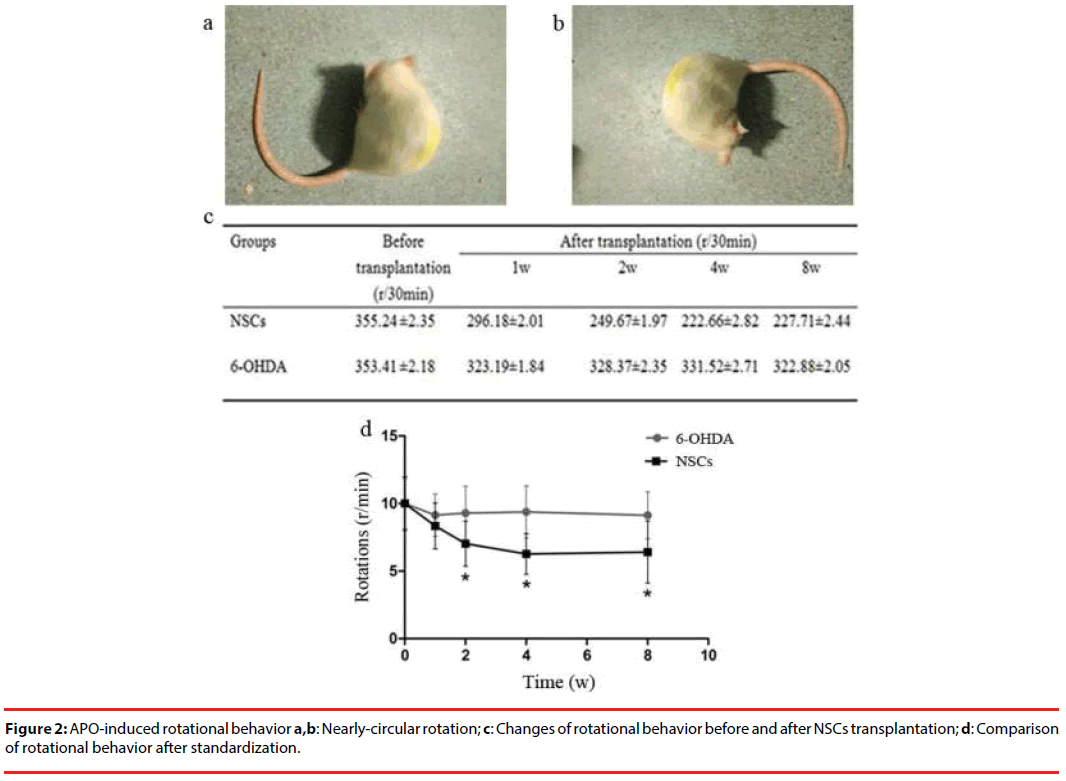
On the first day after the 6-OHDA administration, some rats showed low vitality with signs of piloerection. Over time the rats became tardy and easy to catch with distinct piloerection. The body tilted on one side and the tail was stiff, indicating symptomatic appearance of PD, whereas the control group rats however showed none of the above-mentioned problems. The APO-induced rotational tests demonstrated that the PD rats used the leftward (opposite side of the focal damage) rear legs as the supporting point, spinning end to end on the spot. The rotation rates of the transplanted group began to decline a week after the NSCs transplantation, but the difference was not statistical significance compared with pre-transplantation. Two weeks later, rotation rates had a markedly decreased and the difference was of statistical significance. From week 4 to week 8, rotation rates still remained low. On the contrary, the sham group showed evident increases, but there was no difference among weeks 1, 2, 4 and 8 (Figure 2).
▪ TH expression
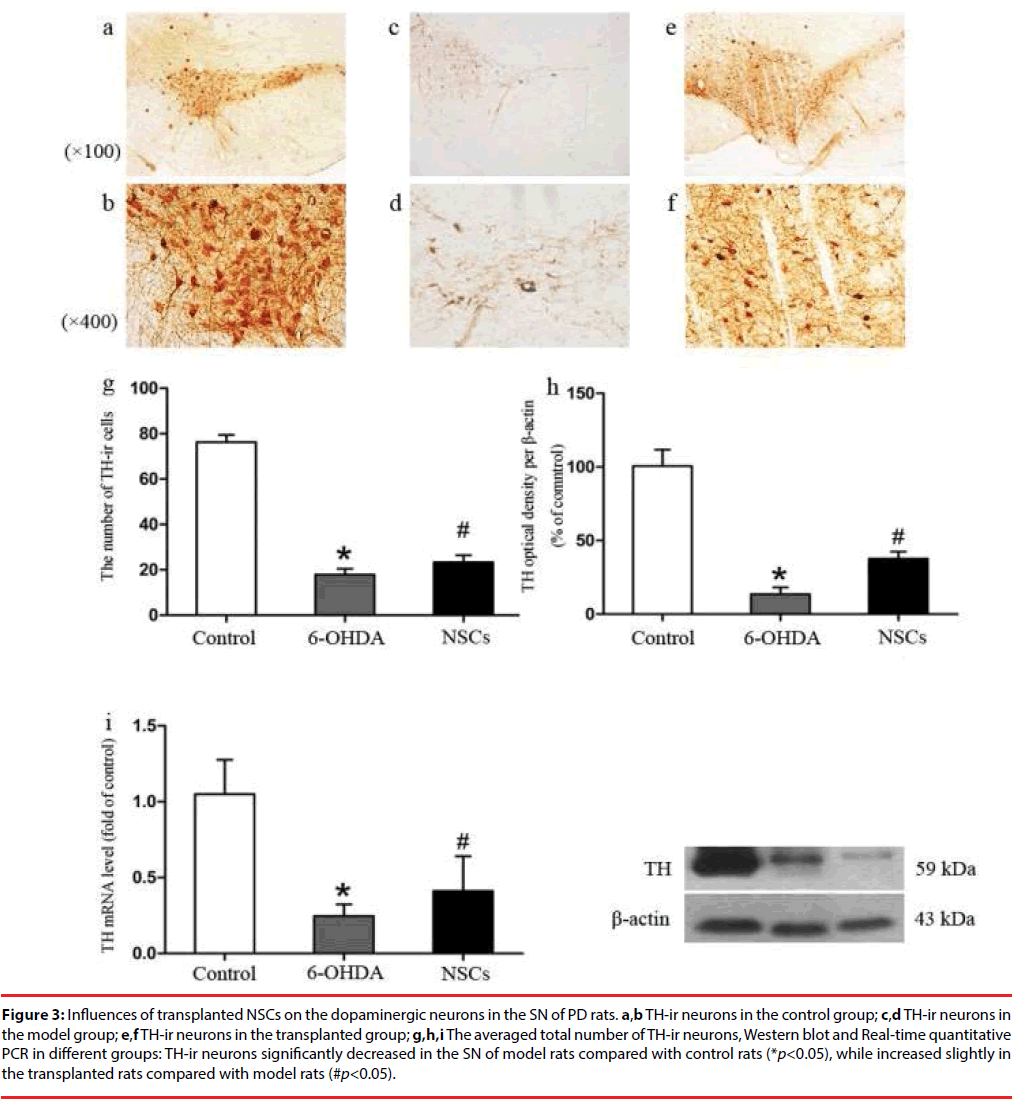
TH-ir neurons in the nigra of the normal SD rats had somata of plump with cytoplasm, multiple dendrites,and high density (total number of cells 76.25 ± 11.00), whereas the cytoplasm of the sham group and the transplant PD groups were thin with decreased dendrites and low density (17.83 ± 9.01, 23.33 ± 10.92 respectively) (Figure 3). Cell counting using the light microscopy revealed that the number of TH-ir neurons in the sham group and the transplant group decreased by 76% and 69% respectively. Compared with the sham group, the number of TH positive cells in the transplant group was 31% higher (Figure 3g), and the levels of TH gene (Figure 3i) and protein expression (Figure 3h) rose slightly, but were far from levels in the control.
Figure 3: Influences of transplanted NSCs on the dopaminergic neurons in the SN of PD rats. a,b TH-ir neurons in the control group; c,d TH-ir neurons in the model group; e,f TH-ir neurons in the transplanted group; g,h,i The averaged total number of TH-ir neurons, Western blot and Real-time quantitative PCR in different groups: TH-ir neurons significantly decreased in the SN of model rats compared with control rats (*p<0.05), while increased slightly in the transplanted rats compared with model rats (#p<0.05).
Discussions
Current treatment for PD is successful for the partial relief of symptoms, but not successful for the basic control of vital etiology. Until now the main therapeutic approaches available are mainly key strategies, including L-DOPA replacement therapy, DA receptor agonist and deep brain stimulation procedures [21]. However as the disease progresses, the pharmacotherapeutic efficacy decreases, and worse still, is complicated by the side effects of motor fluctuation as well as L-DOPA induced dyskinesia. These problems have propelled the shift to cell replacement therapy, therefore a promising restorative therapy intending to secure a long-lasting relief of patients’ symptoms. Various stem cell lines of multifarious origins have been established, which can be further categorized into embryonic stem cells (ESCs), neural stem cells (NSCs), induced neural stem cells (iNSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs). We have shown here that NSCs feature the specialties of strong self-proliferation and differentiation into multiple neurons. These have the potentials to compensate for the lost neurocytes and reduce the symptoms of neurodegenerative diseases such as PD [20]. The low oxygen content in PD midbrain can up-regulate the expression of hypoxiainducible factor (HIF-1) to stimulate the NSCs proliferation, to promote the differentiation of NSCs into dopamine neurons and the secretion of dopamine. It also increases the expression of erythropoietincytin (EPO) and vascular endothelial growth factor (VEFG). This improves the hypoxia-ischemia status and helps to protect the grafted NSCs and the remaining midbrain dopaminergic neurons [21]. It is likely that exogenous transplantation of NSCs plays a vital role in rebuilding PD nigra-striatum circuits and retarding the progression of the diseases. What’s more, the proliferation ability and the neuronal plasticity of NSCs appear to be directly linked to the pathological severity and progression of the disease [22-24].
TH is the enzyme marker of dopaminergic neural specificity. TH-ir neurons are not only an indicator of the level of dopaminergic neurons, but also the directional differentiation of the grafted NSCs as well [7]. Another study has shown that [11]: the TH positive cells in the PD rat nigra clearly decreased, and that when exogenous stem cells were transplanted to the striatum they were able to survive and differentiated into the TH positive cells which secreted the dopaminergic neurotransmitters. In our study, we found that after the neural stem cells were grafted into the PD rat nigra, the number of the TH positive cells increased significantly (by 31%) compared with the sham group, but fell well short of the normal level. As is mentioned in the related literature [24] over 50% of the new neurons face cell apoptosis. What is promising, however, is that even a small amount of TH positive cells differentiated from the grafted NSCs were sufficient to improve the symptoms of PD mobility caused by the abnormal nigrastriatum passage [25]. Only a few (0.018%) of the neural precursor cells (hESCs-NPs), which were derived from the human fetal stem cells and grafted into the striatum differentiated into only a small quantity of TH positive cells (0.018%), but improved remarkably the abnormal behavior of PD rat [26-31]. Our study confirmed that the transplanted NSCs in the nigra also improve the PD rat rotational behaviors, indicating that exogenous NSCs were able to multiply in the PD pathological microenvironment and was protective of the remaining dopaminergic neurons.
To conclude, exogenous NSCs are transplanted into PD rat nigra and partly divide into dopaminergic neurons that attenuate the abnormal rotational behavior. More research is needed to investigate whether the neonatal dopaminergic neurons contribute to promoting/rebuilding the nigra-striatum circuit and secrete dopaminergic neurotransmitters to act as revolutionary therapy of PD.
Acknowledgment
WXP is funded by National Natural Foundation of China (81071065# and 81671103#).
This paper is supported by National Natural Foundation of China (81071065# and 81671103# to WXP).
References
- Zou J, Chen Z, Liang C, et al. Trefoil Factor 3, Cholinesterase and Homocysteine: Potential Predictors for Parkinson's disease Dementia and Vascular Parkinsonism Dementia in Advanced Stage. Aging. Dis 9(1), 51-65 (2018).
- Stocchi F. Therapy for Parkinson's disease: what is in the pipeline? Neurotherapeutics 11(1), 24-33 (2014).
- Wu B, Han L, Sun BM, et al. Influence of deep brain stimulation of the subthalamic nucleus on cognitive function in patients with Parkinson's disease. Neurosci. Bull 30(1), 153-161 (2014).
- Liu J, Li Q, Zhou DY, et al. Experimental study of AADC and NURR1 gene combined with C17.2 neural stem cell transplantation for the treatment of Parkinson's disease. Chin. J. Neuropsychiatric. Dis 31(6), 460-462 (2005).
- Sundberg M, Isacson O. Advances in stem-cell--generated transplantation therapy for Parkinson's disease. Expert. Opin. Biol. Ther 14(4), 437-453 (2014).
- Cave JW, Wang M, Baker H. Adult subventricular zone neural stem cells as a potential source of dopaminergic replacement neurons. Front. Neurosci 8(1), 16 (2014).
- Ziavra D, Makri G, Giompres P, et al. Neural stem cells transplanted in a mouse model of Parkinson's disease differentiates to neuronal phenotypes and reduce rotational deficit. CNS. Neurol. Disord. Drug. Targets 11(7), 829-835 (2012).
- Sun C, Zhang H, Li J, et al. Modulation of the major histocompatibility complex by neural stem cell-derived neurotrophic factors used for regenerative therapy in a rat model of stroke. J. Transl. Med. 8(1), 77 (2010).
- Hermann DM, Peruzzotti-Jametti L, Schlechter J, et al. Neural precursor cells in the ischemic brain - integration, cellular crosstalk, and consequences for stroke recovery. Front. Cell. Neurosci 8(1), 291 (2014).
- Zhu Q, Ma J, Yu L, et al. Grafted neural stem cells migrate to substantia nigra and improve behavior in Parkinsonian rats. Neurosci. Lett 462(3), 213-218 (2009).
- Kang X, Xu H, Teng S, et al. Dopamine release from transplanted neural stem cells in Parkinsonian rat striatum in vivo. Proc. Natl. Acad. Sci. USA 111(44), 15804-15809 (2014).
- Paxinos G, Watson C. The rat brain stereotaxic coordinates: Australia: Academic Press (2006).
- Frau L, Morelli M, Simola N. Performance of movement in hemiparkinsonian rats influences the modifications induced by dopamine agonists in striatal efferent dynorphinergic neurons. Exp. Neurol. 247(1), 663-672 (2013).
- Du XX, Xu HM, Jiang H, et al. Curcumin protects nigral dopaminergic neurons by iron-chelation in the 6-hydroxydopamine rat model of Parkinson's disease. Neurosci. Bull 28(3), 253-258 (2012).
- Wang J, Xu H, Jiang H, et al. Neurorescue effect of rosmarinic acid on 6-hydroxydopamine- lesioned nigral dopamine neurons in rat model of Parkinson's disease. J. Mol. Neurosci 47(1), 113-119 (2012).
- Lee JY, Kim SH, Ko AR, et al. Therapeutic effects of repetitive transcranial magnetic stimulation in an animal model of Parkinson's disease. Brain. Res 1537(1), 290-302 (2013).
- Lv Z, Jiang H, Xu H, et al. Increased iron levels correlate with the selective nigral dopaminergic neuron degeneration in Parkinson's disease. J. Neural. Transm 118(3), 361-369 (2011).
- Li Y, Sun Y, Yang J, et al. Age-dependent dopaminergic dysfunction following fetal exposure to atrazine in SD rats. Environ. Toxicol. Pharmacol 37(3), 1275-1282 (2014).
- Li L, Holscher C, Chen BB,et al. Hepcidin treatment modulates the expression of divalent metal transporter-1, ceruloplasmin, and ferroportin-1 in the rat cerebral cortex and hippocampus. Biol. Trace. Elem. Res 143(3), 1581-1593 (2011).
- Yamashita T, Abe K. Direct reprogrammed neuronal cells as a novel resource for cell transplantation therapy. Cell. Transplant 23(4-5), 435-439 (2014).
- Shen Y, Huang J, Liu L, et al. A compendium of preparation and application of stem cells in Parkinson's disease: current status and future prospects. Front. Aging. Neurosci 8(1), 117 (2016).
- Krabbe C, Bak ST, Jensen P, et al. Influence of oxygen tension on dopaminergic differentiation of human fetal stem cells of midbrain and forebrain origin. PLoSOne 9(5), e96465 (2014).
- Doorn KJ, Drukarch B, van Dam AM , et al. Hippocampal proliferation is increased in presymptomatic Parkinson's disease and due to microglia. Neural. Plast 2014(1), 959154 (2014).
- Sun ZH, Lai YL, Zeng WW, et al. Neural stem/progenitor cells survive and differentiate better in PD rats than in normal rats. Acta. Neurochir. Suppl 87(1), 169-174 (2003).
- Kirkeby A, Nolbrant S, Tiklova K, Heuer A, et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-Based therapy for Parkinson's disease. Cell. Stem. Cell 20(1), 135-148 (2017).
- Shen Y, Huang J, Liu L, et al. A Compendium of Preparation and Application of Stem Cells in Parkinson's disease: Current Status and Future Prospects. Front. Aging. Neurosci 8(1), 117 (2016).
- Ben-Hur T, Idelson M, Khaner H, et al. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem. Cells 22(7): 1246-1255 (2004).
- Xiao JJ, Yin M, Wang ZJ, Wang XP. Transplanted Neural Stem Cells: Playing a Neuroprotective Role by Ceruloplasmin in the Substantia Nigra of PD Model Rats?. Oxid. Med. Cell. Longev 2015(1), 618631 (2015).
- Wei XB, Yan RH, Chen ZY, et al. Combined Diffusion Tensor Imaging and Arterial Spin Labeling as Markers of Early Parkinson’s disease. Sci. Rep 6(1), 33762 (2016).
- Wei X, Gao H, Zou, et al. Contra-directional coupling of Nur77 and Nurr1 in Neurodegeneration: A novel mechanism for Memantine-induced anti-inflammation and anti-mitochondrial impairment. Mol. Neurobiol 53(9), 5876-5892 (2016).
- Xu Y, Yan J, Zhou P, et al. Neurotransmitter receptors and cognitive dysfunction in Alzheimer's disease and Parkinson's disease. Prog. Neurobiol 97(1), 1-13 (2012).