Research Article - Pharmaceutical Bioprocessing (2018) Volume 6, Issue 2
Clinical efficacy of astragalus injection combined with anti-tuberculosis drugs on children with pulmonary tuberculosis complicated with type II diabetes and its influence on serum levels of LEP and APN
- *Corresponding Author:
- Liuxin Wang
Respiratory medicine
The frist people’s hospital
Jining city, 272000, China
E-mail: guandrngcan@163.com
Abstract
Objective: To investigate the clinical efficacy of astragalus injection combined with antituberculosis drugs on children with pulmonary tuberculosis complicated with diabetes and its influence on serum levels of leptin (LEP) and adiponectin (APN).
Methods: 90 cases of children with newly diagnosed smear positive pulmonary tuberculosis complicated with type II diabetes treated in our hospital from February 2015 to February 2017 were selected as the research objects and randomly divided into control group and observation group through random number table method, with 45 cases in each group. All the children were given subcutaneous insulin injection to control blood sugar after admission. The control group was treated with anti-tuberculosis regimen of 2HREZ/4HR while the observation group was treated with astragalus injection on the basis of the control group. Blood glucose, serum levels of LEP and APN, and negative conversion rates of sputum bacteria were detected before and after treatment. The changes of lung lesions and cavities were evaluated by X-ray and CT. Safety was observed as well.
Results: Before treatment, there was no significant difference in the levels of FPG, HbA1c and 2hPG between two groups (P>0.05); After treatment, the levels of FPG, HbA1c and 2hPG were significantly lower than those before treatment in both groups (P<0.05), and the levels decreased more in observation group (P<0.05). After treatment, the serum level of LEP was significantly decreased (P<0.05) while the level of APN was significantly increased (P<0.05) in both groups. Compared with control group, the serum level of LEP was significantly lower (P<0.05) while the level of APN was significantly higher in observation group (P<0.05). After treatment, there were no changes in peripheral T lymphocyte subsets in control group (P>0.05), however, in observation group, the levels of CD3+, CD4+ and CD4+/CD8+ were significantly increased (P<0.05), and the level of CD8+ was significantly decreased (P<0.05). After treatment, the levels of the levels of CD3+, CD4+ and CD4+/CD8+ in observation group were significantly higher than those in control group (P<0.05), and the level of CD8+ was significantly lower than that in control group (P<0.05). At the end of 1 month, 3 months and 6 months after treatment, the sputum negative conversion rate of observation group was significantly higher than that of control group (P<0.05). At the end of 6 months after treatment, the changes of lung lesions and the changes of cavities were better in observation group than those in control group (P<0.05).
Conclusion: Astragalus injection combined with anti-tuberculosis drugs could effectively improve the blood glucose level and immune function, reduce the level of LEP, improve the level of APN, and promote the absorption of tuberculosis focus and sputum negative conversion in children with pulmonary tuberculosis complicated with type II diabetes.
Keywords
pulmonary tuberculosis, type II diabetes, astragalus injection, anti-tuberculosis drugs, sputum negative conversion rate, serum leptin, adiponectin
Introduction
Pulmonary Tuberculosis (PTB) and Diabetes Mellitus (DM) are common and frequent diseases in clinic. PTB is a chronic infectious disease caused by reduced cellular immune response, while DM can lead to an obvious decrease in immune response. Therefore, clinical treatment of children with PTB complicated with DM is difficult to achieve ideal results [1]. These children are characterized with serious infection of lung tissue lesions, high positive rate of sputum bacteria and strong infectivity, and have become an important source of tuberculosis infection [2]. When children are complicated with DM, the recurrence rate of PTB can be obviously increased. When the treatment efficacy of DM is poor, the incidence of active PTB in children is also increasing significantly [3]. It is complicated when PTB is complicated with DM and the risk of adverse outcomes such as tuberculosis treatment failure, death and relapse will increase exponentially [4]. Good glycemic control and prolonged chemotherapy are the key to the treatment of PTB combined with DM. Malnutrition and systemic inflammatory response are found in children with PTB complicated with DM, and the nutritional status is closely related to the severity of the disease [5]. Leptin (LEP) and Adiponectin (APN) play an important role in the nutritional status, immune response and inflammatory reaction of the body. Simple anti-tuberculosis drugs in children with PTB combined with T2DM have the disadvantages of easy recurrence, unsatisfactory lesion and cavity changes [6]. Therefore, it is necessary to find a more effective treatment plan. Combination therapy is the main trend in the treatment of children with PTB combined with DM. Astragalus membranaceous is a traditional Chinese medicine which can invigorate Qi, and astragalus injection is refined from the effective component extracted from Astragalus membranaceous by advanced technology. It has many pharmacological effects, such as strengthening heart, reducing blood sugar, antitumor, protecting liver, enhancing immune function and increasing white blood cell [7]. There are few studies on the clinical efficacy of Astragalus combined with anti-tuberculosis drugs and on the blood glucose, serum LEP and APN levels in the treatment of children with PTB combined with DM. This study was to investigate the clinical efficacy of astragalus injection combined with anti-tuberculosis drugs on children with PTB complicated with DM and its influence on serum levels of LEP and APN, in order to reveal the value of this treatment regimen.
Data and methods
General information
90 cases of children with newly diagnosed smear positive pulmonary tuberculosis complicated with diabetes treated in our hospital from February 2015 to February 2017 were selected as the research objects. The baseline characteristics: 50 males, 40 females; aged 9~16 y, mean age (13.5 ± 0.8) y; course of DM:1-3 years, average course of DM: (1.5 ± 0.2) y.
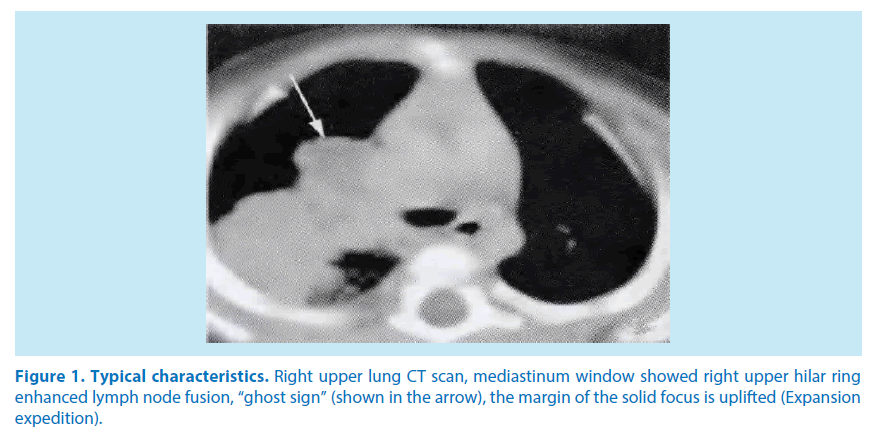
Diagnostic criteria: DM in accordance with the “guidelines for the prevention and treatment of type 2 diabetes China (2013 Edition)”: HbA1c≥6.5%, FPG≥7.0 mmol/L, 2hPG≥11.1 mmol/L; PTB in accordance with the “guide of the diagnosis and treatment of pulmonary tuberculosis”: Cough, expectoration≥14 d, chest imaging characteristics change (Figure 1), positive sputum culture.
Figure 1. Typical characteristics. Right upper lung CT scan, mediastinum window showed right upper hilar ring enhanced lymph node fusion, “ghost sign” (shown in the arrow), the margin of the solid focus is uplifted (Expansion expedition).
Inclusive criteria: all accorded with the clinical diagnostic criteria of DM and PTB; susceptibility test of sputum bacteria was sensitive to primary and second-line anti tuberculosis drugs; the medical ethics Association of our hospital passed the examination and approval; and patients or the family members signed the informed consent; volunteered to participate in the study.
Exclusive criteria: severe acute or chronic complications; other lung diseases; history of immunosuppressive agents taken 3 months before admission; intolerance to anti-tuberculosis drugs or Astragalus injection. The patients were randomly divided into control group and observation group through random number table method, with 45 cases in each group. There were no significant differences in baseline characteristics between the two groups in terms of gender, age, and duration of DM (P>0.05).
Therapeutic method
Neutrul Insulin Injection (Nuo Heling, Novo Nordisk company) was given, the initial dose was 0.2 U/ (kg - D), subcutaneous injection 30 min before meals. The dose of insulin was adjusted according to bedtime and before meals blood glucose levels to maintain ideal and stability. The control group received 2HREZ/4HR chemotherapy (enhanced 2 months, 4 months to consolidate treatment): Isoniazid Tablets (H) 0.3 g, 1 times daily oral; Rifampicin Capsules (R) 0.45 g, 1 times daily oral; ethambutol hydrochloride tablets (E) 0.75 g, 1 times daily oral; Pyrazinamide Tablets (Z) 0.5 g, 3 times a day, oral.
The observation group was given Astragalus Injection (Pharmaceutical Group Limited) on the basis of control group, intravenous infusion, 20 mL 5% Sodium Chloride Injection +150 ml Astragalus injection, 1 /d, 14 d for 1 course, 6 courses.
Observation index
Blood sugar
The fasting blood glucose (FPG), glycosylated hemoglobin (HbA1c) and postprandial 2 h blood glucose (2hPG) levels were detected before and after treatment with OLYMPUSAU5400 automatic biochemical analyzer (OLYMPUS Optical Co., Japan) in two groups.
Serum levels of LEP and APN
LEP and APN levels were detected by enzyme-linked immunosorbent assay (ELISA). The detection kit was provided by Nanjing gold Yibai Biotechnology Co. Ltd. The specific steps of ELISA are as follows:
i. The specific antibody is attached to the solid support to form a solid phase antibody: the unbound antibody and impurities are washed away.
ii. Adding test specimens: make contact with the solid phase antibody and react for a period of time, so that the antigen in the specimen can bind to the solid phase carrier antibody to form a solid phase antigen complex. Wash to remove other unbound material.
iii. Add enzyme-labeled antibody: the antigen on the solid phase immune complexes combine with enzyme-labeled antibody. Thoroughly wash unbound ELISA antibody. The amount of enzyme present on the solid support at this point is positively correlated with the amount of substance being tested in the sample.
iv. Add substrate: enzymes in a sandwich complex catalyze substrate to become a colored product. The qualitative or quantitative determination of the antigen is based on the degree of color reaction.
Peripheral T lymphocyte subsets
Peripheral blood T lymphocyte subsets (CD3+, CD4+, CD8+ and CD4+/CD8+ were detected by On-Chip Sort flow cytometry before and after treatment.
Conversion rate of sputum bacteria
At the end of first, third, sixth months after treatment, 3 consecutive sputum smear acid fast bacilli were examined, and the number of positive cases turned negative, and there was no recurrence.
Lesion condition
Changes of lung lesions were evaluated at the end of the 6 month through X-ray. Obviously, lesion absorption than 1/2; absorption, absorption of lesions less than 1/2; no change, no change in focus; deterioration, lesions increased or new lesions.
Cavity condition
Changes o Cavity were evaluated at the end of the 6 month through HRCT. Closure, Scar or obstruction healed (or disappeared); Narrow, narrow diameter of the cavity is larger than 1/2; No change, hole diameter reduced less than 1/2; increasing hole diameter increases more than 1/2.
Statistical methods
SPSS 21 was used for statistical analysis, measurement data of normal distribution were shown as Mean ± SD and compared by paired t test; Enumeration data represented by rate (%), X2 test was adopted. Grade data represented by rate (%), and rank sum (Z) test was adopted. P<0.05 meaned the difference was statistically significant.
Results
Comparison of blood glucose levels before and after treatment between two groups
Before treatment, there was no significant difference in the levels of FPG, HbA1c and 2hPG between two groups (P>0.05); After treatment, the levels of FPG, HbA1c and 2hPG were significantly lower than those before treatment in both 2 groups (P<0.05), and the levels decreased more in observation group (P<0.05), as shown in Table 1.
| Group | n | FPG (mmol/L) | 2hPG (mmol/L) | HbA1c (%) | |||
|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment |
After treatment | ||
| Control group | 45 | 7.79 ± 0.93 | 6.65 ± 0.54 | 12.04 ± 1.05 | 10.75 ± 1.37 | 6.68 ± 0.81 | 6.14 ± 0.58 |
| Observation group | 45 | 7.81 ± 0.76 | 5.28 ± 0.47 | 12.05 ± 1.13 | 9.94 ± 1.22 | 6.67 ± 0.79 | 5.52 ± 0.43 |
| t | - | 0.305 | 5.799 | 0.623 | 7.005 | 0.132 | 6.032 |
| p | - | >0.05 | <0.05 | > 0.05 | <0.05 | >0.05 | <0.05 |
Table 1. Comparison of blood glucose levels before and after treatment between two groups
Comparison of serum levels of LEP and APN before and after treatment between two groups
The normal reference value of LEP and APN is 0.69-3.61 ug/L and 10.09-16.53 ug/L. After treatment, the serum level of LEP was significantly decreased (P<0.05) while the level of APN was significantly increased (P<0.05) in both groups. Compared with control group, the serum level of LEP was significantly lower (P<0.05) while the level of APN was significantly higher in observation group (P<0.05), as shown in Table 2.
| Group | n | LEP (μg/L) | APN (μg/L) | ||
|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | ||
| Control group | 45 | 7.49 ± 0.67 | 4.52 ± 0.37 | 7.82 ± 0.95 | 11.96 ± 1.15 |
| Observation group | 45 | 7.51 ± 0.62 | 3.87 ± 0.26 | 7.84 ± 1.01 | 14.72 ± 1.34 |
| t | - | 0.187 | 5.657 | 0.246 | 7.564 |
| p | - | >0.05 | <0.05 | >0.05 | <0.05 |
Table 2. Comparison of serum levels of LEP and APN before and after treatment between two groups
Comparison of peripheral T lymphocyte subsets before and after treatment between the two groups
The normal reference value of CD3+, CD4+, CD8+ and CD4+/CD8+ is 61-85%, 28-58%, 19- 48% and 1.4-2.0 respectively. After treatment, there were no changes in peripheral T lymphocyte subsets in control group (P>0.05), however, in observation group, the levels of CD3+, CD4+ and CD4+/CD8+ were significantly increased (P<0.05), and the level of CD8+ was significantly decreased (P<0.05). After treatment, the levels of the levels of CD3+, CD4+ and CD4+/CD8+ in observation group were significantly higher than those in control group (P<0.05), and the level of CD8+ was significantly lower than that in control group (P<0.05), as shown in Table 3.
| Groups | n | CD3+(%) | CD4+(%) | CD8+ (%) | CD4+/CD8+ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||
| Control group | 45 | 36.72 ± 3.25 | 37.15 ± 2.69 | 25.71 ± 2.47 | 26.84 ± 3.15 | 24.15 ± 2.46 | 23.21 ± 1.78 | 1.13 ± 0.16 | 1.19 ± 0.62 |
| Observation group | 45 | 36.73 ± 3.48 | 49.56 ± 2.57 | 25.73 ± 2.62 | 35.09 ± 54.02 | 24.21 ± 2.66 | 17.96 ± 1.02 | 1.14 ± 0.15 | 1.61 ± 0.23 |
| t | - | 0.464 | 5.754 | 0.354 | 6.709 | 0.121 | 8.675 | 0.265 | 6.126 |
| p | - | >0.05 | <0.05 | > 0.05 | <0.05 | >0.05 | <0.05 | >0.05 | <0.05 |
Table 3. Comparison of peripheral T lymphocyte subsets before and after treatment between the two groups
Comparison of sputum negative conversion rate between two groups
At the end of 1 month, 3 months and 6 months after treatment, the sputum negative conversion rate of observation group was significantly higher than that of control group (P<0.05), as shown in Table 4.
| Group | n | End of 1 month after treatment | End of 3 months after treatment | End of 6 months after treatment |
|---|---|---|---|---|
| Control group | 45 | 13 (28.9) | 28 (62.2) | 37 (82.2) |
| Observation group | 45 | 27 (60.0) | 36 (80.0) | 44 (97.8) |
| X2 | - | 5.013 | 5.985 | 6.526 |
| p | - | <0.05 | <0.05 | <0.05 |
Table 4. Comparison of sputum negative conversion rate between two groups [n(%)]
Comparison of lung lesions between two groups
At the end of 6 months after treatment, the changes of lung lesions were better in observation group than those in control group (P<0.05), as shown in Table 5.
| Group | n | Significant absorption | Absorption | No change | Deterioration |
|---|---|---|---|---|---|
| Control group | 45 | 20 (44.4) | 11 (24.4) | 10 (22.2) | 4 (8.9) |
| Observation group | 45 | 35 (77.8) | 7 (15.6) | 2 (4.4) | 1 (2.2) |
| Z | - | -2.036 | |||
| P | - | <0.05 | |||
Table 5. Comparison of lung lesions between two groups [n(%)]
Comparison of cavities between two groups
At the end of 6 months after treatment, the changes of cavities were better in observation group those that in control group (P<0.05), as shown in Table 6.
| Group | n | Closure | Reduce | No change | Increase |
|---|---|---|---|---|---|
| Control group | 45 | 12 (26.7) | 19 (42.2) | 8 (17.8) | 6 (13.3) |
| Observation group | 45 | 34 (75.6) | 9 (20.0) | 1 (2.2) | 1 (2.2) |
| Z | - | -2.942 | |||
| P | - | <0.05 | |||
Table 6. Comparison of cavities between two groups [n(%)]
Summary
Before treatment, there was no significant difference in the levels of FPG, HbA1c and 2hPG between two groups (P>0.05); After treatment, the levels of FPG, HbA1c and 2hPG were significantly lower than those before treatment in both 2 groups (P<0.05), and the levels decreased more in observation group (P<0.05). After treatment, the serum level of LEP was significantly decreased (P<0.05) while the level of APN was significantly increased (P<0.05) in both groups. Compared with control group, the serum level of LEP was significantly lower (P<0.05) while the level of APN was significantly higher in observation group (P<0.05). After treatment, there were no changes in peripheral T lymphocyte subsets in control group (P>0.05), however, in observation group, the levels of CD3+, CD4+ and CD4+/CD8+ were significantly increased (P<0.05), and the level of CD8+ was significantly decreased (P < 0.05). After treatment, the levels of the levels of CD3+, CD4+ and CD4+/CD8+ in observation group were significantly higher than those in control group (P<0.05), and the level of CD8+ was significantly lower than that in control group (P<0.05). At the end of 1 month, 3 months and 6 months after treatment, the sputum negative conversion rate of observation group was significantly higher than that of control group (P<0.05). At the end of 6 months after treatment, observed group were significantly better than the control group (P<0.05), the observation group of cavity change was significantly better than the control group (P<0.05). The changes of lung lesions were better in observation group than those in control group (P<0.05), and the changes of cavities were better in observation group those that in control group (P<0.05). These results suggested that Astragalus injection combined with anti-tuberculosis drugs could effectively improve the blood glucose level and immune function, reduce the level of LEP, improve the level of APN, and promote the absorption of tuberculosis focus and sputum negative conversion in children with pulmonary tuberculosis complicated with diabetes.
Discussion
Recent years, DM and tuberculosis are two kinds of chronic diseases which have serious threat to human health. At present, the prevalence of tuberculosis in China is gradually decreasing, but with the improvement of living standards, the prevalence of diabetes is increasing gradually. In clinic, the two diseases often merge into existence. The incidence of PTB complicated with DM is increasing year by year, which has become a disease of clinical concern. At present, the treatment effect and the prognosis are poor for the children with pulmonary tuberculosis complicated with diabetes [8]. The recommended treatment for PTB in China is 2HREZ/4HR or 2H3R3Z3E3/4H3R3 [9]. Astragalus membranaceous is a traditional Chinese medicine which can invigorate Qi, and astragalus injection is refined from the effective component extracted from Astragalus membranaceus by advanced technology. It has many pharmacological effects, such as strengthening heart, reducing blood sugar, anti-tumor, protecting liver, enhancing immune function and increasing white blood cell. CD4+ cells are a group of T lymphocytes with physiological functions of auxiliary induction. After sensitization, CD4+ cells can synthesize and release TNF-, IL-2, chemokines and other mediators, promote the rapid proliferation of macrophages and kill Mycobacterium tuberculosis, and significantly improve the cellular immune response physiological function. CD8+ cells are a group of T lymphocytes which can inhibit the cytotoxic physiological function and can effectively inhibit the physiological function of cellular immune response in vivo [10]. When the proportion of CD4+ cells decreases significantly, it is difficult to limit the Mycobacterium tuberculosis to the lesion. However, when the ratio of CD8+ cells is relatively increased, the physiological function of dissolved Mycobacterium tuberculosis is significantly enhanced, which causes the complete exposure of Mycobacterium tuberculosis, and the phenomena of a large number of release and diffusion [11,12]. Due to the high proportion of CD8 + cells, tuberculosis children can synthesize and release inhibitory factors, resulting in the reduction of CD4 + cells, thereby weakening their physiological function. In our study, after treatment, there were no changes in peripheral T lymphocyte subsets in control group (P>0.05), however, in observation group, the levels of CD3+, CD4+ and CD4+/CD8+ were significantly increased (P<0.05), and the level of CD8+ was significantly decreased (P<0.05). After treatment, the levels of the levels of CD3+, CD4+ and CD4+/CD8+ in observation group were significantly higher than those in control group (P<0.05), and the level of CD8+ was significantly lower than that in control group (P<0.05). These showed that Astragalus injection combined with 2HREZ/4HR anti-tuberculosis drugs could increase the serum total number of white blood cells, promote the phagocytosis and bactericidal ability of neutrophils and macrophages, enhance cellular immunity, and become one of the effective ways to treat PTB combined with DM.
As shown in our study, after treatment, the levels of FPG, HbA1c and 2hPG were significantly lower than those before treatment in both 2 groups (P<0.05), and the levels decreased more in observation group (P<0.05). Sputum negative conversion rate, lesion absorption rate and cavity closure rate in observation group were significantly higher than those in control group (P<0.05)’ Therefore, astragalus injection combined with 2HREZ/4HR anti tuberculosis drugs in the treatment of children with PTB complicated with DM has significant curative effect, which can significantly improve the negative conversion rate of sputum bacteria, focus absorption rate and cavity closure rate.
LEP is a protein hormone involved in the regulation of sugar, fat and energy metabolism, and is an important indicator of the nutritional status of the body [13].
LEP resistance is a risk factor for DM, and as a marker of metabolism and inflammation, it decreases in PTB. Low levels of LEP play an important role in maintaining the stability of the environment during PTB and are closely related to the regulation of energy metabolism and activation of inflammatory response [14]. APN is an insulin sensitizing hormone. It is an important factor in the regulation of lipid metabolism and blood glucose homeostasis, and has the potential of anti DM, atherosclerosis and inflammation. High level of APN can improve insulin sensitivity, so it can reduce the incidence of DM. Mycobacterium tuberculosis has a pathway of cholesterol metabolism, which means it can accumulate and use cholesterol in the body, invade macrophages and thereby infecting the human body. At the same time, high blood lipid level in DM provides the condition for its growth. LEP and APN regulate the nutritional status of the body through the regulation of body nutrition metabolism, thereby affecting the occurrence and development of PTB combined with DM [15]. In this study, astragalus injection combined with 2HREZ/4HR anti tuberculosis drugs can significantly regulate the balance of LEP and APN in the body, adjust the immune regulation and nutritional status of the body, thus enhancing the therapeutic effect of the disease.
In conclusion, Astragalus injection combined with anti-tuberculosis drugs could effectively improve the blood glucose level and immune function, reduce the level of LEP, improve the level of APN, and promote the absorption of tuberculosis focus and sputum negative conversion in children with pulmonary tuberculosis complicated with diabetes. Therefore, it was worth popularizing and applying.
References
- Herrera Barrios MT, Torres Rojas M, Juárez Carvajal E et al. Molecular mechanisms of the immune response in human pulmonary tuberculosis. Revista. Del. Instituto. Nacional. De. Enfermedades. Respiratorias. 18(4), 327-336 (2005).
- Subbian S, Tsenova L, Kim MJ et al. Lesion-specific immune response in granulomas of patients with pulmonary tuberculosis: a pilot study. Plos. One. 10(7), e0132249 (2015).
- Banda H, Kang'Ombe C, Harries AD et al. Mortality rates and recurrent rates of tuberculosis in patients with smear-negative pulmonary tuberculosis and tuberculous pleural effusion who have completed treatment. Int. J. Tuber. Lung. Dis. 4(10), 968-74 (2000).
- Chaudhry LA, Essa EB, Al-Solaiman S et al. Prevalence of diabetes type-2 & pulmonary tuberculosis among Filipino and treatment outcomes: A surveillance study in the Eastern Saudi Arabia. Int. J. Mycobacteriol. 1(2), 106-109 (2012).
- Gilsantana L, Almeidajunior JL, Oliveira CA et al. Diabetes is associated with worse clinical presentation in tuberculosis patients from brazil: a retrospective cohort study. Plos. One. 11(47), 5539-5545 (2016).
- Van ZS, Marais BJ, Hesseling AC et al. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int. J. Tuber. Lung. Dis. 10(1), 13-18 (2006).
- He Y, Tang C, Luo B et al. Study on influence of astragalus and its injection on insulin, blood glucose and blood lipid in patients with coronary heart disease. Modern. Diag. Treat. 16(4), 221-223 (2005).
- Lauer JA, Williams BG, Gojka R et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC. Public. Health. 7(1), 234 (2007).
- Gu J, Tang S J, Tan SY et al. An open-label, randomized and multi-center clinical trial to evaluate the efficacy of Silibinin in preventing drug-induced liver injury. Int. J. Clin. Exp. Med. 8(3), 4320-4327 (2015).
- Grotzke JE,Lew DM. Role of CD8 + T lymphocytes in control of mycobacterium tuberculosis infection. Microbes. Infect. 7(4), 776-788 (2005).
- Toulza F, Tsang L, Ottenhoff TH et al. Mycobacterium tuberculosis-specific CD4(+) T-cell response is increased, and Treg cells decreased, in anthelmintic-treated patients with latent TB. Eur. J. Immunol. 46(3), 752-761 (2015).
- Riou C, Gray CM, Lugongolo M et al. A Subset of Circulating Blood Mycobacteria-Specific CD4 T Cells Can Predict the Time to Mycobacterium tuberculosis Sputum Culture Conversion. Plos. One. 9(7), e102178 (2014).
- Pérez-Montarelo D, Rodríguez MC, Fernández A et al. Haplotypic diversity of porcine LEP, and LEPR, genes involved in growth and fatness regulation. J. Appl. Genetics. 56(4), 1-9 (2015).
- Bienertovávasků J, Dostálová Z, Kanková K et al. Is there any link between severe pre-eclampsia and defined polymorphisms in leptin and adiponectin genes. J. Obstetrics. Gynaecol. Res. 34(5), 858-864 (2008).
- Luo HL, Li RH. Effect of electroacupuncture on leptin and adiponectin in simple obesity patients. Zhen. Ci. Yan. Jiu. 32(4), 264-267 (2007).